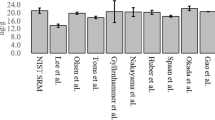
Abstract
The laboratory of the present author is a participant of Randox International Quality Assessment Scheme (RIQAS). The author receives twenty six samples for thirty nine general chemistry analysis per year, reports of which are to be sent fortnightly. Amongst thirty nine analytes there are partial lipid profile parameters like total cholesterol and triglycerides. So, the author decided to run a pilot study of full lipid profile from these samples. At the first phase four samples from two different cycles were considered as test materials. The reports of these samples were quite satisfactory. The trial run was given for fifteen consecutive days. (Presented by the author in the Annual Conference of ACBI, 2004).
The success of trial runs for fifteen days proved that EQA samples from RIQAS may be used for full lipid profile analysis. Results were compared against Randox internal quality control samples (IQA) and were found to be accurate and reproducible.
Similar content being viewed by others
References
Pal, Shyamali (2002) Estimation of CKMB from RIQAS control serum at 37 degree Celsius. Ind. J. Clin. Biochem. 17, 88–90.
Pal, Shyamali (2003) Estimation of CKMB from RIQAS control serum at 25 and 30 degree Celsius. R. G. Kar Medical Bulletin 9, 8–11.
Myers G.L., Kimberly M.M., Waymack P.P., Smith S.J., Cooper G.R. and Sampson E.J. (2000) A reference method laboratory network for cholesterol: a model for standardization and improvement of clinical laboratory measurements. Clin. Chem. 46, 1762–1772.
Henry, J.B. (1991) Clinical diagnosis and management by laboratory methods, 18th Ed. W.B. Saunders, Philadelphia, P. 204–211.
Hoang, M.P., Hirany, S.V., Parupia, J., Devaraj, S. and Jialal, I. (1998) Comparison of two homogeneous high density lipoprotein cholesterol assays. Arch. Patrol. Lab. Med. 122, 1005–1009.
Nakamura, M., Tanigati, Y., Yamamoto, M., Hino, K. and Manabe, M. (1997) Homogeneous assay of serum LDL cholesterol on an automatic analyzer. Clin. Chem. 43/6, S.260–261.
RIQAS external quality assessment reports, general clinical chemistry reports of cycle 29, (RQ9123), RIQAS H.Q., Crumlin, U.K.
Friedewald, W.T., Levy, R.I. and Frederickson, D.S. (1972) Estimation of the concentration of low density lipoprotein cholesterol in plasma without use of the preparative ultraentrifuge. Clin. Chem. 18, 499–502.
Hoffmann, G.E., Schleilcher, E., Weiss, L. and Hoffmann, S. (1982) Comparison of two methods for very low density and low density lipoprotein cholesterol determination. J. Clin. Chem. Clin. Biochem. 20, 457–460.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pal, S. Study of lipid profile from RIQAS quality control sample. Indian J Clin Biochem 20, 18–23 (2005). https://doi.org/10.1007/BF02867395
Issue Date:
DOI: https://doi.org/10.1007/BF02867395