Abstract
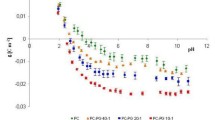
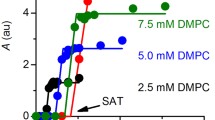
Liposomes were prepared from mixed micelles by a dilution method. Mixed micellar solutions, containing constant octyl glucoside and egg phosphatidylcholine concentrations and varying amounts of cholesterol and/or a charged compound, were diluted at defined rates. After dilution, the resulting liposome dispersions were sequentially concentrated, washed or dialysed, and filtered. The effect of lipid composition and experimental conditions on physicochemical characteristics was studied. Fairly homogeneous liposome dispersions with mean diameters ranging from 100 to over 200 nm could be obtained. The particle size was dependent on cholesterol content and surface charge, and could be reproducibly controlled by adjustment of the dilution rate. Liposomes with a mean diameter below 100 nm could also be obtained, but were heterodisperse and unstable. The incorporation of charged compounds was monitored by microelectrophoresis.31P-NMR measurements indicated that the liposomes were unilamellar. Dialysis appeared to be more convenient than washing to remove octyl glucoside.
Similar content being viewed by others
References
Milsmann MHW, Schwendener RA, Weder HG. The preparation of large single bilayer liposomes by a fast and controlled dialysis. Biochim Biophys Acta 1978;512:147–55.
Rhoden V, Goldin SM. Formation of unilamellar lipid vesicles of controllable dimensions by detergent dialysis. Biochemistry 1979;18:4173–6.
Zumbuehl O, Weder HG. Liposomes of controllable size in the range of 40 to 180 nm by defined dialysis of lipid/detergent mixed micelles. Biochim Biophys Acta 1981;640:252–62.
Schwendener RA, Asanger M, Weder HG.n-Alkylglucosides as detergents for the preparation of highly homogeneous bilayer liposomes of variable sizes (60–240 nm Ø) applying defined rates of detergent removal by dialysis. Biochem Biophys Res Commun 1981;100:1055–62.
Brunner J, Skrabal P, Hauser H. Single bilayer vesicles prepared without sonication. Physico-chemical properties. Biochim Biophys Acta 1976;455:322–31.
Enoch HG, Strittmatter P. Formation and properties of 1000-Å-diameter, single bilayer phospholipid vesicles. Proc Natl Acad Sci USA 1979;76:145–9.
Allen TM, Romans AY, Kercret H, Segrest JP. Detergent removal during membrane reconstitution. Biochim Biophys Acta 1980;601:328–42.
Ueno M, Tanford C, Reynolds JA. Phospholipid vesicle formation using nonionic detergents with low monomer solubility. Kinetic factors determine vesicle size and polydispersity. Biochemistry 1984;23:3070–6.
Schurtenberger P, Mazer N, Waldvogel S, Känzig W. Preparation of monodisperse vesicles with variable size by dilution of mixed micellar solutions of bile salt and phosphatidylcholine. Biochim Biophys Acta 1984; 775:111–4.
Fischer TH, Lasic DD. A detergent depletion technique for the preparation of small vesicles. Mol Liq Cryst 1984;102:141–53.
Weder HG, Zumbuehl O. The preparation of variably sized homogeneous liposomes for laboratory, clinical, and industrial use by controlled detergent dialysis. In: Gregoriadis G, ed. Liposome Technology. Vol 1. Boca Raton: CRC Press, 1984:79–107.
Eytan GD. Use of liposomes for reconstitution of biological functions. Biochim Biophys Acta 1982;694: 185–202.
Eriksson H, Mattiasson B. In: Gregoriadis G, ed. Liposome Technology. Vol II. Boca Raton: CRC Press, 1984:141–56.
North JR, Morgan AJ, Thompson JL, Epstein MA. Purified Epstein-Barr Virus Mr 340,000 glycoprotein induces potent virus-neutralizing antibodies when incorporated in liposomes. Proc Natl Acad Sci USA 1982;79:7504–8.
Jiskoot W, Teerlink T, Van Hoof MMM, et al. Immunogenic activity of gonococcalPi in mice with three different lipoidal adjuvants delivered in liposomes and in complexes. Infect Immun (in press).
Kagawa Y, Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XXV. Reconstitution of vesicle catalyzing32Pi-adenosine triphosphatase exchange. J Biol Chem 1971;246:5477–87.
Helenius A, Fries E, Kartenbeck J. Reconstitution of Semliki Forest Virus membrane. J Cell Biol 1977;75:866–80.
Simons K, Sarvas M, Garoff H, Helenius A. Membranebound and secreted forms of penicillinase fromBacillus licheniformis. J Mol Biol 1978;126:673–90.
Petri WA Jr, Wagner RR. Reconstitution into liposomes of the glycoprotein of vesicular stomatitis virus by detergent dialysis. J Biol Chem 1979;254:4313–6.
Mimms LT, Zampighi G, Nozaki Y, Tanford C, Reynolds A. Phospholipid vesicle formation and transmembrane protein incorporation using octyl glucoside. Biochemistry 1981;20:833–40.
Casali P, Sissons JGP, Fujinami RS, Oldstone BA. Purification of measle virus glycoprotein and their integration into artificial lipid membranes. J Gen Virol 1981;54:161–71.
Helenius A, Sarvas M, Simons K. Asymmetric and symmetric membrane reconstitution by detergent elimination. Eur J Biochem 1981;116:27–35.
Jackson ML, Litman BJ. Rhodopsin-egg phosphatidylcholine reconstitution by an octyl glucoside dilution procedure. Biochim Biophys Acta 1985;812:369–76.
Racker E, Chien TF, Kandrach A. A cholate-dilution procedure for the Ca2+ pump,32Pi-ATP exchange, and oxidative phosphorylation. FEBS Lett 1975;57:14–8.
Serrano R, Kanner BI, Racker E. Purification and properties of the proton-translocating adenosine triphosphatase complex of bovine heart mitochondria. J Biol Chem 1976;251:2453–61.
Racker E, Violand B, O'Neal S, Alfonzo M, Telford J. Reconstitution, a way of biochemical research; some new approaches to membrane-bound enzymes. Arch Biochem Biophys 1979;198:470–7.
Juliano RL, Stamp D. The effect of particle size and charge on the clearance rates of liposomes and liposome encapsulated drugs. Biochem Biophys Res Comm 1975;63:651–8.
Latif N, Bacchawat BK. Liposomes in immunology. J Biol Sci 1984;6:491–502.
Shinoda K, Yamaguchi T, Hori R. The surface tension and the critical micelle concentration in aqueous solution of β-d-alkyl glucosides and their mixtures. Bull Chem Soc Jpn 1961;34:237–41.
Stubbs GW, Smith HG Jr, Litman BJ. Alkyl glucosides as effective solubilizing agents for bovine rhodopsin. A comparison with several commonly used detergents. Biochim Biophys Acta 1975;425:46–56.
Bartlett JR. Phosphorus assay in column chromatography. J Biol Chem 1959;234:466–8.
Böttcher CJF, Van Gent CM, Pries C. A rapid and sensitive sub-microphosphorus determination. Anal Chim Acta 1961;24:203–4.
Crommelin DJA. Influence of lipid composition and ionic strength on the physical stability of liposomes. J Pharm Sci 1983;73:1559–63.
Mazer NA, Benedek GB, Carey MC. Quasielastic light-scattering studies of aqueous biliary lipid systems. Mixed micelle formation in bile salt-lecithin solutions. Biochemistry 1980;19:601–15.
Schurtenberger P, Mazer NA, Känzig W. Dynamic laser light scattering studies of the micelle to vesicle transition in model and native bile. Hepatology 1984;4:143S-7S.
Lasic DD. A molecular model for vesicle formation. Biochim Biophys Acta 1982;692:501–2.
Mazer NA, Kwasnick RF, Carey MC, Benedek GB. Quasielastic light scattering spectroscopic studies of aqueous bile salt, bile salt-lecithin and bile salt-lecithin-cholesterol solutions. In: Mittal KL, ed. Micellisation, Solubilisation and Microemulsions. Vol 1. New York: Plenum Press, 1977:383–402.
Fromherz P. Lipid-vesicle structure: size control by edge-active agents. Chem Phys Lett 1983;94:259–66.
Helfrich W. The size of bilayer vesicles generated by sonication. Phys Lett 1974;50A:115–6.
Fromherz P, Rüppel D. Lipid vesicle formation: the transition from open disks to closed shells. FEBS Lett 1985;179;155–9.
Poznanski MJ, Juliano RL. Biological approaches to the controlled delivery of drugs: a critical review. Pharmacol Rev 1984;36:277–336.
Jackson ML, Schmidt CF, Lichtenberg D, Litman BJ, Albert AD. Solubilization of phosphatidylcholine bilayers by octyl glucoside. Biochemistry 1982;21:4576–82.
Israelachvili JN. Theoretical considerations on the asymmetric distribution of charged phospholipid molecules on the inner and outer layers of curved bilayer membranes. Biochim Biophys Acta 1973;23:659–63.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jiskoot, W., Teerlink, T., Beuvery, E.C. et al. Preparation of liposomes via detergent removal from mixed micelles by dilution. Pharmaceutisch Weekblad Scientific Edition 8, 259–265 (1986). https://doi.org/10.1007/BF01960070
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01960070