Abstract
Cases of human infection with a novel H7N9 avian influenza virus (AIV) were first reported in March 2013, which caused 115 deaths within a single year. Beyond that, other subtypes of H7 AIV were isolated from poultry in eastern China during the same period, including H7N7 and H7N2 AIV. In the present study, a subtype H3N2 AIV was isolated from ducks from Anhui Province, China. Sequence and phylogenetic analyses revealed that seven gene segments of this virus showed the highest sequence homology with that of the H7 subtype influenza virus, which is presumed to be the reassortants of the H3 and H7 subtypes AIV. The present study also reconfirmed that the reassortment between the H7 subtype and waterfowl-originating AIVs universally occurred in waterfowl. Animal inoculation tests showed that the virus has low pathogenicity in chickens; however, it could be replicated in the lungs of mice. The emergence of this H3N2 isolate emphasizes the importance of enhancing the surveillance of waterfowl-originating AIVs, the identification of novel reassortant strains, and characterization of their biological properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Waterfowl are well known as a nature reservoir pool for avian influenza viruses (AIV), these birds usually do not show any symptoms while carrying the viruses [1, 2]. Reassortment between influenza viruses is an important route for increasing gene diversity and acquiring antigenic shift, which in turn enables the influenza virus to acquire the capacity to establish infection across various species, thereby subsequently causing a pandemic [3].
H3 is one of the most epidemic subtypes of influenza viruses that triggered the 1968 Hong Kong pandemic through gene reassortment with the human H2N2 virus [4]. A triple-reassortant H3N2 influenza virus occurred in swine populations in the mid-1990s, which eventually became the prevalent strain in North America [5, 6]. In 2004, an equine-originating H3N8 influenza A virus was transmitted to the canine population [7]. Subsequently, a canine influenza epidemic caused by an avian-originating H3 influenza virus was reported [8]. Domestic ducks serve as an ideal environment for the reassortment of the H3 subtype influenza with other subtypes, which plays an important role in the ecology of AIV and may potentially be a threat to human health [9].
In the present study, we isolated a strain of H3N2 AIV from a domestic duck during a routine surveillance in Anhui Province, China. The molecular and biological properties of the virus were systemically analyzed. The results of the present study extends our understanding of the genetic diversity of the H3 subtype AIV; it also raises concern about the generation of novel viruses that may cause interspecies transmission, as well as improve measures in responding to future pandemics.
Materials and methods
Virus isolation
In February 2014, a total of 111 oropharyngeal swabs were collected from domestic chickens and ducks of a live poultry market in Anhui Province, which were then kept in phosphate buffered saline (PBS) supplemented with 2 × 106 U/L penicillin and 2 × 106 U/L streptomycin. The samples were transported to the laboratory within 24 h (h). The samples were centrifuged at 4000×g for 5 min, and the supernatants were used to inoculate 10-day-old embryonated specific pathogen-free (SPF) chicken eggs. The presence of AIV was detected using hemagglutination (HA) and hemagglutination inhibition (HI) assays, and then confirmed by reverse transcription-polymerase chain reaction (RT-PCR), RT-PCR was performed using specific primers, F: 5′-GTTAAAGCGATCATATTT-3′, R: 5′-CTTTGTCTGCAGCGTACCCACT-3′. Only a H3N2 virus was isolated from oropharyngeal swabs of ostensibly healthy ducks. The virus isolate was named A/duck/Anhui/D293/2014. The 50 % egg infection dose (EID50) of the virus was determined in 10-day-old embryonated eggs using the method of Reed and Muench.
Genome sequence and phylogenetic analysis
Viral RNA was extracted from the allantoic fluid by using the TRIzol LS reagent (Life Technologies, Rockville, MD, USA) and then reverse transcribed with a 12-nucleotide primer, 5′-AGCAAAAGCAGG-3′. PCR was conducted using gene-specific primers for each viral gene [10], and subsequently, the PCR products for all eight segments of the virus were gel purified using a QIAquick PCR purification kit (Qiagen, Valencia, CA, USA). Direct Sanger sequencing was performed by the Beijing Genomics Institute, China. Sequence data were compiled using the SEQMAN program (DNASTAR, Madison, WI, USA). Multiple sequence alignment and the phylogenetic and molecular evolutionary analyses were conducted using the neighbor-joining method, with 1000 bootstrap replicates using the software, MEGA, version 6.0.
Pathogenicity in chickens
One group of 6 six-week-old chickens were intranasally inoculated with 0.1 mL of 106 EID50 of the virus. Three of the chickens were euthanized at day 4 post-inoculation (p.i.), and their lung, trachea, spleen, kidney, brain, and intestine were collected for viral titration. The lung tissues were also used in histological examination. To monitor virus shedding, tracheal and cloacal swabs were collected from the other three chickens on days 2, 4, and 6 p.i., for virus titration in eggs. Another group of 10 six-week-old SPF chickens were intravenously injected with 0.1 mL of a 1:10 dilution of bacteria-free allantoic fluid containing the virus. Clinical signs of disease or death of the chickens were monitored and used in the calculation of intravenous pathogenicity index (IVPI).
Pathogenicity in mice
To determine the virulence of this isolate in mice, 6 six-week-old female BALB/c mice were lightly anesthetized and intranasally inoculated with 50 μL of the virus at an EID50 of 106. Three of the mice were euthanized on day 3 p.i., and tissues samples including the lung, brain, spleen, and kidney were collected for virus titration and histopathological evaluation. The other three mice were monitored daily for 14 days for weight loss and mortality. Three mice inoculated with PBS were used as control.
Results
Phylogenetic analysis and molecular characterization of the H3N2 virus
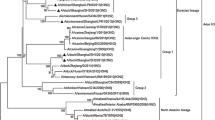
All eight gene segments of this domestic duck-origin H3N2 virus was sequenced and compared with the sequences of representative influenza virus sequences retrieved from GenBank for phylogenetic reconstruction. The phylogenetic tree of the HA gene showed that the H3N2 strain clustered in the Eurasian lineage of the H3 subtype AIV, with a highest similarity to the A/duck/Zhejiang/D1-2/2013(H3N6) strain. The N2 gene of this isolate shared the highest nucleotide homology (97.5 %) with the H7N2 AIV A/duck/Wenzhou/775/2013 strain (Fig. 1). The phylogenetic trees of internal genes also belonged to the Eurasian lineage and were mainly derived from the H7N7 and H7N3 AIVs. Polymerase basic proteins 1 and 2 (PB1 and PB2) had the highest genetic identities with the A/duck/Huzhou/4227/2013(H7N7) strain, and polymerase acidic protein (PA) was highly similar to that of the A/duck/Huzhou/4268/2013(H7N7) strain. The nucleoprotein (NP) and matrix protein (M) showed the highest identities with those of the A/duck/Zhejiang/2/2011(H7N3) and A/duck/Huzhou/3916/2013(H7N3) strains, respectively. The nonstructural (NS) protein possessed the highest nucleotide homologies with that of the A/mallard/Korea/NHG187/2008(H7N7) strain (Table 1, Figure S1). The eight segments of this isolate showed the highest sequence homology with different AIV subtypes from domestic ducks and mallards in eastern China and Korea, indicating these areas were undergoing a high frequency of reassortment involving the H7 subtype and waterfowl-originating AIVs.
Phylogenetic relationships of HA and NA genes of isolate A/duck/Anhui/D293/2014(H3N2). The phylogenetic tree was prepared by the distance-based neighbor-joining method in software MEGA 4.1. The reliability of the tree was assessed by bootstrap analysis of 1000 replicates. Horizontal distances are proportional to genetic distance. Viruses isolated in this study are indicated in red (Color figure online)
The HA gene of this H3N2 isolate harbored a sequence motif, PEKQTR/GLF, at its cleavage site, which was characteristic of low-pathogenic AIVs [11]. Amino acids at the receptor binding site were well conserved, and the mutations, Q226S and G228S, were not detected in the HA protein, which suggested that this strain preferentially recognizes an α-2,3-linked sialic acid [12, 13]. The H3N2 virus has six potential N-glycosylation sites at positions 24, 38, 54, 181, 301, and 499 in the HA protein. No mutations were observed in the NA and M2 proteins, which are responsible for resistance to NA inhibitors (oseltamivir and zanamivir) or amantadines [14]. The mutations, E627K and D701N, in the PB2, which is responsible for the cross-species transmission of AIVs, were not observed [15]. A previous study revealed that the S42P and D92E substitutions in the NS1 protein enhance the resistance of AIVs to interferons (IFNs) [16]. However, this H3N2 virus harbored no mutation at residues 42 and 92 of the NS1 protein.
Pathogenicity in chickens
All the six chickens intravenously inoculated with H3N2 virus did not show any clinical signs or death during the 14-day observation period. The IVPI value for this H3N2 isolate was 0. The virus was detected in the intestines and kidneys of the intranasally inoculated chickens on day 4 p.i. at low virus titer levels. The challenged chickens showed no obvious histopathological lesions. Viral shedding in tracheal swabs in all three chickens at days 4 and 6 p.i. showed low virus titer levels (Fig. 2). Cloacal swabs indicated viral shedding in one chicken on day 6 p.i. All three chickens demonstrated seroconversion in HI assays performed at 14 days p.i.
Viral replication of A/duck/Anhui/D293/2014(H3N2) in chickens. The viral titers found in tracheal and cloaca swabs were determined on days 2, 4, and 6 p.i. The values shown are means ± standard deviations. Virus shedding were shown as virus positive birds/test birds above each bar. The dashed line indicates the lower limit of detection (Color figure online)
Pathogenicity in mice
The H3N2 influenza virus commonly occurs in swine and humans [17]. In the present study, we evaluated the potential of the A/duck/Anhui/D293/2014(H3N2) strain in infecting a mammalian host. All experimental mice showed slightly ruffled fur from days 2 to 6 p.i. and maintained the weight after a slight drop at day 6 p.i. Mice in the mock group showed no weight loss or clinical signs during the 14-day observation period. Low level of virus replication was detected in all the three mice’s lungs (<1.75 EID50/mL). The virus was not detected in the other organs, including the brain, spleen, and kidney. The challenged mice showed no obvious lung histopathological lesions on day 3 p.i.
Discussion
H3 subtype influenza viruses extensively circulate among various bird and mammalian species and thus pose a threat to public health [4]. The antigenicity and biological characteristics extensively vary between H3 subtype influenza viruses from different host species. However, this continues to cause interspecies transmission over time [7, 8]. The inherent high mutation rate of the influenza virus has resulted in extensive changes in the biological characteristics of the H3 AIV, thus prompting proactive research investigations on this matter.
The H7 subtype can cause fowl plague in poultry and result in a pandemic [18, 19]. In 2013, the novel avian-originating H7N9 influenza A virus, which was first reported in eastern China, has caused two outbreaks in the human population and caused over a hundred deaths from 2013 to 2014 [20]. Simultaneously, several influenza virus subtypes, H7N7, H7N3, and H7N2, were isolated from domestic chickens and ducks across five provinces in eastern China [21, 22]. In the present study, a novel H3N2 AIV, A/duck/Anhui/D293/2014, was isolated from apparently healthy ducks from a live poultry market in eastern China, which is a high-prevalence area for influenza [23]. The NA gene of the virus showed the highest homology with A/duck/Wenzhou/775/2013(H7N2), which was isolated in 2013 in Wenzhou, Zhejiang Province [21]. All its polymerase genes were derived from H7N7 AIVs that were isolated in 2013 from Zhejiang Province [22]. These findings suggest that this H3N2 isolate was a natural recombinant virus of H7 and waterfowl-derived H3 subtype AIVs. Although it has been proven to be a low-pathogenic AIV in chickens and mice, the virus could replicate in these species at low levels. It could therefore act as a donor for the exchange of gene segments with that of the H7 subtype influenza virus, which poses a huge potential risk based on its capacity to cross the species barrier and subsequently infect humans. The high frequency of reassortment events between H7 subtype and waterfowl-originating AIV that have occurred in eastern China raises concern regarding the generation of a novel virus that may cause a pandemic similar to the novel avian H7N9 influenza virus [20].
In summary, we have described an H3N2 AIV isolated from a domestic duck from a live poultry market situated in Anhui Province, eastern China. Its phylogenetic and molecular characteristics, as well as virulence in chickens and mice were examined. Our study emphasizes the need to enhance surveillance efforts in monitoring the evolution of AIVs in eastern China to effectively control further spread of new viruses.
All the sequences obtained in this study have been deposited in GenBank with the accession numbers KT022352 to KT022359.
References
D.J. Alexander, A review of avian influenza in different bird species. Vet. Microbiol. 74, 3–13 (2000)
R.G. Webster, W.J. Bean, O.T. Gorman, T.M. Chambers, Y. Kawaoka, Evolution and ecology of influenza A viruses. Microbiol. Rev. 56, 152–179 (1992)
W.G. Laver, The origin of pandemic strains of influenza. Evidence from studies of the structure of the hemagglutinin subunits. Adv. Exp. Med. Biol. 31, 29–46 (1972)
C. Scholtissek, W. Rohde, V. Von Hoyningen, R. Rott, On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology 87, 13–20 (1978)
N.N. Zhou, D.A. Senne, J.S. Landgraf, S.L. Swenson, G. Erickson, K. Rossow, L. Liu, K. Yoon, S. Krauss, R.G. Webster, Genetic reassortment of avian, swine, and human influenza A viruses in American pigs. J. Virol. 73, 8851–8856 (1999)
N.N. Zhou, D.A. Senne, J.S. Landgraf, S.L. Swenson, G. Erickson, K. Rossow, L. Liu, K.J. Yoon, S. Krauss, R.G. Webster, Emergence of H3N2 reassortant influenza A viruses in North American pigs. Vet. Microbiol. 74, 47–58 (2000)
P.C. Crawford, E.J. Dubovi, W.L. Castleman, I. Stephenson, E.P. Gibbs, L. Chen, C. Smith, R.C. Hill, P. Ferro, J. Pompey, R.A. Bright, M.J. Medina, C.M. Johnson, C.W. Olsen, N.J. Cox, A.I. Klimov, J.M. Katz, R.O. Donis, Transmission of equine influenza virus to dogs. Science 310, 482–485 (2005)
D. Song, B. Kang, C. Lee, K. Jung, G. Ha, D. Kang, S. Park, B. Park, J. Oh, Transmission of avian influenza virus (H3N2) to dogs. Emerg. Infect. Dis. 14, 741–746 (2008)
G. Deng, D. Tan, J. Shi, P. Cui, Y. Jiang, L. Liu, G. Tian, Y. Kawaoka, C. Li, H. Chen, Complex reassortment of multiple subtypes of avian influenza viruses in domestic ducks at the Dongting Lake Region of China. J. Virol. 87, 9452–9462 (2013)
E. Hoffmann, J. Stech, Y. Guan, R.G. Webster, D.R. Perez, Universal primer set for the full-length amplification of all influenza A viruses. Arch. Virol. 146, 2275–2289 (2001)
D.A. Steinhauer, Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology 258, 1–20 (1999)
M. Matrosovich, A. Tuzikov, N. Bovin, A. Gambaryan, A. Klimov, M.R. Castrucci, I. Donatelli, Y. Kawaoka, Early alterations of the receptor-binding properties of H1, H2, and H3 avian influenza virus hemagglutinins after their introduction into mammals. J. Virol. 74, 8502–8512 (2000)
H. Wu, N. Wu, X. Peng, C. Jin, X. Lu, L. Cheng, H. Yao, L. Li, Molecular characterization and phylogenetic analysis of H3 subtype avian influenza viruses isolated from domestic ducks in Zhejiang Province in China. Virus Gen. 49, 80–88 (2014)
H.K. Lee, J.W. Tang, T.P. Loh, A.C. Hurt, L.L. Oon, E.S. Koay, Molecular surveillance of antiviral drug resistance of influenza A/H3N2 virus in Singapore, 2009–2013. PLoS ONE 10, e0117822 (2015)
Z. Li, H. Chen, P. Jiao, G. Deng, G. Tian, Y. Li, E. Hoffmann, R.G. Webster, Y. Matsuoka, K. Yu, Molecular basis of replication of duck H5N1 influenza viruses in a mammalian mouse model. J. Virol. 79, 12058–12064 (2005)
P. Jiao, G. Tian, Y. Li, G. Deng, Y. Jiang, C. Liu, W. Liu, Z. Bu, Y. Kawaoka, H. Chen, A single-amino-acid substitution in the NS1 protein changes the pathogenicity of H5N1 avian influenza viruses in mice. J. Virol. 82, 1146–1154 (2008)
L. Jennings, Avian influenza: a public health risk for New Zealand. N. Zeal. Med. J. 117, U843 (2004)
J.A. Belser, O. Blixt, L.M. Chen, C. Pappas, T.R. Maines, N. Van Hoeven, R. Donis, J. Busch, R. McBride, J.C. Paulson, J.M. Katz, T.M. Tumpey, Contemporary North American influenza H7 viruses possess human receptor specificity: implications for virus transmissibility. Proc. Natl. Acad. Sci. 105, 7558–7563 (2008)
J.A. Belser, X. Lu, T.R. Maines, C. Smith, Y. Li, R.O. Donis, J.M. Katz, T.M. Tumpey, Pathogenesis of avian influenza (H7) virus infection in mice and ferrets: enhanced virulence of Eurasian H7N7 viruses isolated from humans. J. Virol. 81, 11139–11147 (2007)
R. Gao, B. Cao, Y. Hu, Z. Feng, D. Wang, W. Hu, J. Chen, Z. Jie, H. Qiu, K. Xu, X. Xu, H. Lu, W. Zhu, Z. Gao, N. Xiang, Y. Shen, Z. He, Y. Gu, Z. Zhang, Y. Yang, X. Zhao, L. Zhou, X. Li, S. Zou, Y. Zhang, X. Li, L. Yang, J. Guo, J. Dong, Q. Li, L. Dong, Y. Zhu, T. Bai, S. Wang, P. Hao, W. Yang, Y. Zhang, J. Han, H. Yu, D. Li, G.F. Gao, G. Wu, Y. Wang, Z. Yuan, Y. Shu, Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 368, 1888–1897 (2013)
T.T. Lam, J. Wang, Y. Shen, B. Zhou, L. Duan, C.L. Cheung, C. Ma, S.J. Lycett, C.Y. Leung, X. Chen, L. Li, W. Hong, Y. Chai, L. Zhou, H. Liang, Z. Ou, Y. Liu, A. Farooqui, D.J. Kelvin, L.L. Poon, D.K. Smith, O.G. Pybus, G.M. Leung, Y. Shu, R.G. Webster, R.J. Webby, J.S. Peiris, A. Rambaut, H. Zhu, Y. Guan, The genesis and source of the H7N9 influenza viruses causing human infections in China. Nature 502, 241–244 (2013)
T.T. Lam, B. Zhou, J. Wang, Y. Chai, Y. Shen, X. Chen, C. Ma, W. Hong, Y. Chen, Y. Zhang, L. Duan, P. Chen, J. Jiang, Y. Zhang, L. Li, L.L. Poon, R.J. Webby, D.K. Smith, G.M. Leung, J.S. Peiris, E.C. Holmes, Y. Guan, H. Zhu, Dissemination, divergence and establishment of H7N9 influenza viruses in China. Nature 522, 102–105 (2015)
K.F. Shortridge, Is China an influenza epicentre? Chin. Med. J. 110, 637–641 (1997)
Acknowledgments
This work was supported by the National Basic Research Program (973 Program, 2011CB504702) of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Zhen F. Fu.
Chong Li and Meng Yu contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11262_2016_1323_MOESM1_ESM.pdf
Phylogenetic relationships of PB2, PB1, PA, NP, M and NS genes of isolate A/duck/Anhui/D293/2014(H3N2). The phylogenetic tree was prepared by the distance-based neighbor-joining method in software MEGA 4.1. The reliability of the tree was assessed by bootstrap analysis of 1000 replicates. Horizontal distances are proportional to genetic distance. Viruses isolated in this study are indicated in red. Supplementary material 1 (PDF 270 kb)
Rights and permissions
About this article
Cite this article
Li, C., Yu, M., Liu, L. et al. Characterization of a novel H3N2 influenza virus isolated from domestic ducks in China. Virus Genes 52, 568–572 (2016). https://doi.org/10.1007/s11262-016-1323-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-016-1323-0