Abstract
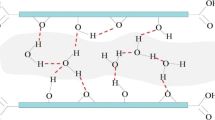
In this review, we outline our computational efforts in understanding the interactions between water and various graphitic carbon surfaces based on quantum-mechanical level calculations. Among them, we have determined the geometry structures, electronic properties, and vibrational infrared and resonant Raman spectra of water clusters on graphene surface and single walled carbon nanotubes (SWCNTs). Specifically, we found that (1) the hexamer water cluster undergoes isomerization when interacting with a graphene surface, while the smaller water clusters maintain their cyclic or linear configurations, with little changes in their infrared peak positions and almost perfect graphene surfaces due to the physical adsorption of the water clusters; and (2) water molecules can form cylindrical crystalline structures, referred to as ice nanotubes, by hydrogen bonding under confinement within SWCNTs. Our computational results are expected to shed light on the graphene and SWCNTs studies and their applications in areas such as biology and materials science.









Similar content being viewed by others
References
A. Hodgson and S. Haq (2009). Surf. Sci. Rep. 64, 381.
M. A. Henderson (2002). Surf. Sci. Rep. 46, 1.
J. Carrasco, A. Hodgson, and A. Michaelides (2012). Nat. Mater. 11, 667.
A. A. Chialvo, L. Vlcek, and P. T. Cummings (2014). J. Phys. Chem. C 118, 19701.
M. Lorenz, B. Civalleri, L. Maschio, M. Sgroi, and D. Pullini (2014). J. Comput. Chem. 35, 1789.
I. Hamada (2012). Phys. Rev. B 86, 195436.
T. Zhang, Q. Xue, S. Zhang, and M. Dong (2012). Nano Today 7, 180.
E. M. Huff and P. Pulay (2009). Mol. Phys. 107, 1197.
C. S. Lin, R. Q. Zhang, S. T. Lee, M. Elstner, T. Frauenheim, and L. J. Wan (2005). J. Phys. Chem. B 109, 14183.
P. U. Andersson, M. T. Suter, N. Markovic, and J. B. C. Pettersson (2007). J. Phys. Chem. C 111, 15258.
C. Feng, R. Q. Zhang, S. L. Dong, T. A. Niehaus, and T. Frauenheim (2007). J. Phys. Chem. C 111, 14131.
R. X. Song, S. Wangmo, M. S. Xin, Y. Meng, P. Huai, Z. G. Wang, and R. Q. Zhang (2013). Nanoscale 5, 6767.
E. G. Gordeev, M. V. Polynski, and V. P. Ananikov (2013). Phys. Chem. Chem. Phys. 15, 18815.
G. Cicero, J. C. Grossman, E. Schwegler, F. Gygi, and G. Galli (2008). J. Am. Chem. Soc. 130, 1871.
B. S. González, J. Hernández-Rojas, J. Bretón, and J. M. G. Llorente (2007). J. Phys. Chem. C 111, 14862.
J. Hernández-Rojas, F. Calvo, J. Bretón, and J. M. G. Llorente (2012). J. Phys. Chem. C 116, 17019.
H. B. Li, L. D. Zou, L. K. Pan, and Z. Sun (2010). Environ. Sci. Technol. 44, 8692.
T. S. Sreeprasad, S. M. Maliyekkal, K. P. Lisha, and T. Pradeep (2011). J. Hazard. Mater. 186, 921.
R. Zangi (2004). J. Phys. 116, S5371.
N. N. Avgul and A. V. Kieslev Chemistry and Physics of Carbon (Dekker, New York, 1970).
R. R. Q. Freitas, R. Rivelino, F. de Brito Mota, and C. M. C. de Castilho (2011). J. Phys. Chem. A 115, 12348.
A. Simon and F. Spiegelman (2013). J. Chem. Phys. 138, 194309.
B. Collignon, P. N. M. Hoang, S. Picaud, and J. C. Rayez (2005). Chem. Phys. Lett. 406, 430.
G. Perez-Hernandez and B. Schmidt (2013). Phys. Chem. Chem. Phys. 15, 4995.
X. Wang, L. Feng, and Z. Cao (2014). Acta. Chim. Sinica. 72, 487.
I. W. Sudiarta and D. J. W. Geldart (2006). J. Phys. Chem. A 110, 10501.
D. Feller and K. D. Jordan (2000). J. Phys. Chem. A 104, 9971.
A. Vernov and W. A. Steele (1992). Langmuir 8, 155.
P. Jurecka, J. Sponer, J. Cerny, and P. Hobza (2006). Phys. Chem. Chem. Phys. 8, 1985.
S. Tsuzuki, K. Honda, T. Uchimaru, M. Mikami, and K. Tanabe (2000). J. Am. Chem. Soc. 122, 11450.
E. Voloshina, D. Usvyat, M. Schuetz, Y. Dedkov, and B. Paulus (2011). Phys. Chem. Chem. Phys. 13, 12041.
M. Elstner, D. Porezag, G. Jungnickel, J. Elsner, M. Haugk, T. Frauenheim, S. Suhai, and G. Seifert (1998). Phys. Rev. B 58, 7260.
B. Aradi, B. Hourahine, and T. Frauenheim (2007). J. Phys. Chem. A 111, 5678.
T. Frauenheim, G. Seifert, M. Elstner, T. Niehaus, C. Kohler, M. Amkreutz, M. Sternberg, Z. Hajnal, A. Di Carlo, and S. Suhai (2002). J. Phys. 14, 3015.
M. Elstner, P. Hobza, T. Frauenheim, S. Suhai, and E. Kaxiras (2001). J. Chem. Phys. 114, 5149.
M. Elstner, T. Frauenheim, and S. Suhai (2003). J. Mol. Struct. 632, 29.
W. J. Fan and R. Q. Zhang (2008). Sci. China Ser. B 51, 1203.
W. J. Fan, R. Q. Zhang, B. K. Teo, B. Aradi, and T. Frauenheim (2009). Appl. Phys. Lett. 95, 013116.
W. J. Fan, J. Zeng, and R. Q. Zhang (2009). J. Chem. Theory Comput. 5, 2879.
C. H. Wang, S. Li, R. Q. Zhang, and Z. J. Lin (2012). Nanoscale 4, 146.
C. H. Wang, Q. Wu, W. J. Fan, R. Q. Zhang, and Z. Lin (2012). Org. Biomol. Chem. 10, 5049.
J. Kim and K. S. Kim (1998). J. Chem. Phys. 109, 5886.
S. S. Xantheas, C. J. Burnham, and R. J. Harrison (2002). J. Chem. Phys. 116, 1493.
K. Karapetian, K. D. Jordan, and J. P. Devlin, Properties of Water Clusters on a Graphite Sheet. in V. Buch (ed.), Water in Confined Environments (Springer, New York, 2003), p 139.
D. R. Paul (2012). Science 335, 413.
M. Whitby and N. Quirke (2007). Nat. Nanotechnol. 2, 87.
R. Zangi and A. E. Mark (2003). Phys. Rev. Lett. 91, 025502.
M. S. Dresselhaus, G. Dresselhaus, and P. C. Eklund Sciences of fullerenes and carbon nanotubes (Academic Press, San Diego, 1996).
M. S. P. Sansom and P. C. Biggin (2001). Nature 414, 156.
N. C. M. Majumder, R. Andrews, and B. J. Hinds (2005). Nature 438, 44.
J. K. Holt, H. G. Park, Y. Wang, M. Stadermann, A. B. Artyukhin, C. P. Grigoropoulos, A. Noy, and O. V. Bakajin (2006). Science 312, 1034.
N. K. Jena, M. K. Tripathy, A. K. Samanta, K. R. S. Chandrakumar, and S. K. Ghosh (2012). Theor. Chem. Acc. 131, 1205.
L. Wang, J. Zhao, F. Li, H. Fang, and J. P. Lu (2009). J. Phys. Chem. C 113, 5368.
B. Yin and S.-L. Dong (2009). Chin. Phys. Lett. 26, 086402.
H. Maniwa, M. Abe, A. Udaka, S. Suzuki, Y. Achiba, H. Kira, K. Matsuda, H. Kadowaki, and Y. Okabe (2005). Chem. Phys. Lett. 401, 534.
K. Koga, G. T. Gao, H. Tanaka, and X. C. Zeng (2001). Nat. Chem. 412, 802.
L. A. Curtiss, D. J. Frurip, and M. J. Lander (1979). Chem. Phys. Lett. 71, 2703.
J. Bai, J. Wang, and X. C. Zeng (2006). Proc. Natl. Acad. Sci. 103, 19664.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, WJ., Zhang, RQ. Water Clusters on Graphitic Carbon Surfaces. J Clust Sci 26, 361–373 (2015). https://doi.org/10.1007/s10876-015-0854-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-015-0854-1