Abstract
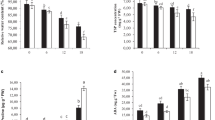
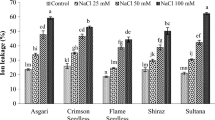
The role of exogenously applied Methyl jasmonate (MeJA) in morphological responses, photochemical efficiency, changes of malondialdehyde content, and the activities of some antioxidant enzymes were investigated in four Iranian grapevine cultivars. MeJA improved morphological traits containing dry and fresh weight, node number, and shoot length. MeJA induced an oxidative stress, as shown by an increase in lipid peroxidation. Activities of catalase, peroxidase, and ascorbate peroxidase were higher in MeJA-treated grapevines than in controls while the relative water content and leaf water loss of grapevine cultivars demonstrated a non-significant difference between the control and varying levels of MeJA. MeJA was positively affected in recovery of the leaf chlorophyll fluorescence (photochemical efficiency) of grapevine cultivars, although the mean proline content of MeJA-treated grapevines indicated a significant decrease when compared with those of the controls. These results suggest that MeJA could act as an intervener in grapevine responses by the enhancing the activity of antioxidants and recovery of photochemical efficiency, leading to enhanced grapevine performance.
Similar content being viewed by others
Abbreviations
- ASC:
-
ascorbate peroxidase
- CAT:
-
catalase
- DW:
-
dry weight
- FW:
-
fresh weight
- LWL:
-
leaf water lose
- MDA:
-
malondialdehyde
- MeJA:
-
methyl jasmonate
- POX:
-
peroxidase
- ROS:
-
reactive oxygen species
- RWC:
-
relative water content
- TBA:
-
thiobarbituric acid
- TCA:
-
trichloroacetic acid
References
Alleweldt G, Spiegel-Roy P, Reisch B. 1990. Grapes (Vitis), In JN Moore, JR Ballington, eds, Genetic Resources of Temperate Fruit and Nut Crops, Acta Hortic. 290: 291–337
Bates LS, Waldern RP, Teare ID. 1973. Rapid determination of free proline from water stress studies. Plant Soil 39: 205–207
Bhardway R, Singhal G. 1981. Effect of water stress on photochemical activity of chloroplast during greening etiolated barley seedlings. Plant Cell Physiol. 22: 155–162
Cheong JJ, Choi YD. 2003. Methyl jasmonate as a vital substance in plants. Trends Genet. 19: 409–413
Chong TM, Abdullah MA, Fadzillah NM, Lai OM, Lajis NH. 2005. Jasmonic acid elicitation of anthraquinones with some associated. Enzyme Microb. Technol. 36: 469–477
Ding CK, Wang CY, Gross KC, Smith DL. 2002. Jasmonate and salicylate induce the expression of athogenesis-related-protein genes and increase resistance to chilling injury in tomato fruit. Planta 214: 895–901
Fang WC, Kao CH. 2001. Inhibition of methyl jasmonatepromoted senescence in rice leaves by a metal chelator, 2, 2′-bipyridine. Plant Growth Regul. 33: 87–93
FAO. 1997. FAO production year book for 1997. 51, FAO, Rome
Fatahi R, Ebadi A, Bassil N, Mehlenbacher SA, Zamani Z. 2003. Characterization of Iranian grapevine cultivars using microsatellite markers. Vitis 42: 185–192
Huguet RV, Sulpice R, Lefort C, Maerskalck V, Emery N, Larher FR. 2003. The suppression of osmoinduced proline response of Brassica napus L. var oleifera discs by polyunsaturated fatty acids and methyl-jasmonate. Plant Sci. 164: 119–127
Hung KT, Kao CH. 1998. Involvement of lipid peroxidation in methyl senescence in detached rice leaves. Plant Growth Regul. 24: 17–21
Hung KT, Kao CH. 2007. The participation of hydrogen peroxide in methyl jasmonate-induced NH4+ accumulation in rice leaves. J. Plant Physiol. 164: 1469–1479
Heil M. 2004. Induction of two indirect defenses benefits lima bean (Phaseolus lunatus, Fabaceae) in nature. J Ecol. 92: 527–536
Jiang MY, Zhang JH. 2002. Water stress-induced abscisic accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J. Exp. Bot. 379: 2401–2410
Jung S. 2004. Effect of chlorophyll reduction in Arabidopsis thaliana by methyl jasmonate or norflurazon on antioxidant systems. Plant Physiol. Biochem. 42: 225–231
Keramat B, Kalantari KM, Arvin MJ. 2009. Effects of methyl jasmonate in regulating cadmium induced oxidative stress in soybean plant (Glycine max L.). Afr. J. Biotechnol. 3: 240–244
Koda Y. 1997. Possible involvement of jasmonates in various morphogenic events. Physiol. Plant. 100: 639–646
Mahmood M, Shirani Bidabadi S, Ghobadi S, Gray DJ. 2012. Effect of methyl jasmonate treatments on alleviation of polyethylene glycol-mediated water stress in banana (Musa accuminata cv. Berangan, AAA) shoot tip cultures. Plant Growth Regul. 68: 161–169
Mata CG, Lamattina L. 2001. Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol. 126: 1196–1204
Meir S, Droby S, Davidson H, Alsevia S, Cohen L, Horev B, Philosoph-Hadas S. 1998. Suppression of Botrytis rot in cut rose flowers by postharvest application of methyl jasmonate. Postharvest Biol. Tech. 13: 235–243
Mittler R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7: 405–410
Mohammad BA, Hahn EJ, Peak Y. 2007. Methyl jasmonate and salicylic acid induced oxidative stress and accumulation of phenolics in Panax ginseng bioreactor root suspension cultures. Molecules 12: 607–621
Nakano Y, Asada YK. 1981. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Photochem. Photobiol. 37: 679–690
Nejatian MA. 2012. Collection and evaluation of grapevine genotypes of the Gazvine. FAO, 28 p
Norastehnia A, Nojavan AM. 2006. Effect of methyl jasmonate on the enzymatic antioxidant defense system in maize seedling subjected to paraquat. Asian J. Plant Sci. 5: 17–23
Parthier B. 1991. Jasmonates, new regulators of plant growth and development: many facts and few hypotheses on their actions. Bot. Acta 104: 446–454
Pospisilova J. 2003. Participation of phytohormones in the stomatal regulation of gas exchange during water stress. Biol. Plant. 46: 491–506
Ravinkar M, Zel J, Plaper I, Spacapan A. 1993. Jasmonic acid stimulates shoot and bulb formation of garlic in vitro. J. Plant Growth Regul. 12: 73–77
Ravinkar, M, Rode J, Gogala N, Benedicic D. 1990. Regulation of organogenesis with jasmonic acid. Acta Hort. 280: 169–172
Rohwer CL, Erwin JE. 2008. Horticultural applications of jasmonates: a review. J. Hort. Sci. Biotechnol. 83: 283–304
Santos I, Salema R. 2000. Promotion by jasmonic acid of bulb formation in shoot cultures of Narcissus triandrus L. Plant Growth Regul. 30: 133–138
Sasaki SY, Taki N, Obayashi T, Aono M, Matsumoto F et al. 2005. Coordinated activation of metabolic pathways for antioxidants and defense compounds by jasmonates and their roles in stress tolerance in Arabidopsis. Plant J. 44: 653–680
Sharifi P, Amirnia R, Majidi E, Hadi H, Roustaii M, Nakhoda B, Alipoor HM, Moradi F. 2012. Relationship between drought stress and some antioxidant enzymes with cell membrane and chlorophyll stability in wheat lines. Afr. J. Microbiol. Res. 6: 617–623
Srinivas ND, Rashmi KR, Raghavarao KSMS. 1999. Extraction and purification of a plant peroxidase by aqueous two-phase extraction coupled with gel filtration. Process Biochem. 35: 43–48
Ueda J, Saniewski M. 2006. Methyl jasmonate-induced stimulation of chlorophyll formation in the basal part of tulip bulbs kept under natural light conditions. J. Fruit. Ornam. Plant Res. 14: 199–210
Velikova V, Loreto F. 2005. On the relationship between isoprene emission and thermo tolerance in Phragmites ausrralis leaves exposed to high temperatures and during the recovery from a heat stress. Plant, Cell Environ. 28: 318–327
Velikova V, Yordanov I, Edreva A. 2000. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Protective role of exogenous polyamines. Plant Sci. 151: 59–66
Wang CY, Buta JG. 1994. Methyl Jasmonate reduces chilling injury in Cucurbita pepo through its regulation of abscisic acid and polyamine levels. Environ. Exp. Bot. 34: 427–432
Wang F, Zeng B, Sun Z, Zhu C. 2009. Relationship between proline and Hg+2 — induced oxidative stress in tolerant rice mutant. Arch. Environ. Contam. Toxicol. 56: 723–731
Wang SY. 1999. Methyl jasmonate reduces water stress in strawberry. J. Plant Growth Regul. 18: 127–134
Wasternack C, Parthier B. 1997. Jasmonate-signaled plant gene expression. Trends Plant Sci. 2: 302–307
Xing H, Tan L, An L, Zhao Z, Wang S, Zhang C. 2004. Evidence for the involvement of nitric oxide and reactive oxygen species in osmotic stress tolerance of wheat seedlings: Inverse correlation between leaf abscisic acid accumulation and leaf water loss. Plant Growth Regul. 42: 61–68
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bidabadi, S.S., Mehri, H., Ghobadi, C. et al. Morphological, physiological and antioxidant responses of some Iranian grapevine cultivars to methyl jasmonate application. J. Crop Sci. Biotechnol. 16, 277–283 (2013). https://doi.org/10.1007/s12892-013-0096-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12892-013-0096-4