Abstract
Introduction
Among patients with chronic obstructive pulmonary disease (COPD), the frequency and severity of past exacerbations potentiates future events. The impact of current therapies on exacerbation frequency and severity in patients with different exacerbation risks is not well known.
Methods
A post hoc analysis of patients at low (≤1 exacerbation [oral steroids/antibiotics requirement] and no COPD-related hospitalization in the year preceding trial entry) or high (≥2 exacerbations [oral steroids/antibiotics requirement] or ≥1 COPD-related hospitalization[s] in the year preceding trial entry) exacerbation risk, from the Prevention of Exacerbations with Tiotropium in Chronic Obstructive Pulmonary Disease (POET-COPD®) database.
Results
Compared with salmeterol, tiotropium significantly increased time to first COPD exacerbation (hazard ratio 0.84; 95% confidence interval [CI] 0.76–0.92; p = 0.0002) and reduced the number of COPD exacerbations (rate ratio 0.90; 95% CI 0.81–0.99; p = 0.0383) in patients at high exacerbation risk. With treatment, the risk of remaining in the high-risk exacerbator subgroup was statistically lower with tiotropium versus salmeterol (risk ratio [RR] 0.89; 95% CI 0.80–1.00; p = 0.0478). For low-risk patients, time to first COPD exacerbation and number of COPD exacerbations were numerically lower with tiotropium versus salmeterol. With treatment, the risk of transitioning from a low to a high exacerbation risk was lower with tiotropium versus salmeterol (RR 0.87; 95% CI 0.71–1.07; p = 0.1968).
Discussion
This analysis confirms the higher efficacy of tiotropium versus salmeterol in prolonging time to first COPD exacerbation and reducing number of exacerbations in patients both at low and high exacerbation risk.
Funding
Boehringer Ingelheim and Pfizer.
Clinical trial registration number: ClinicalTrials.gov NCT00563381.
Similar content being viewed by others
Introduction
Exacerbations have a deleterious effect on the morbidity and mortality of patients with chronic obstructive pulmonary disease (COPD) [1] by increasing hospitalization [2], worsening lung function [3] and health-related quality of life, and decreasing survival [4]. As such, the prevention of exacerbations is an important goal of COPD therapy [5], the importance of which is reflected in the current Global Initiative for Chronic Obstructive Lung Disease (GOLD) document on the management of COPD [6].
The frequency of exacerbations increases in parallel with the severity of COPD [7]. In the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE®) (ClinicalTrials.gov #NCT00292552) observational study of patients with COPD, exacerbation rate was shown to increase with GOLD staging, such that 22% of patients with GOLD Stage II disease had two or more exacerbations during 1 year of follow-up, whereas 47% of patients with GOLD Stage IV disease had frequent exacerbations over the same period. Across all GOLD stages, the single best predictor of future episodes was prior exacerbations [8]. Additionally, the results from the ECLIPSE study suggested that patients with COPD could be phenotyped according to their exacerbation risk. Those patients who experienced fewer than two exacerbations per year were classified as “infrequent exacerbators”; those experiencing two or more per year were classified as “frequent exacerbators” and considered to be different phenotypes [8]. More recently, the GOLD 2013 guidelines revisited this classification of patients at risk for exacerbations and divided patients into low (≤1 exacerbations; no severe exacerbations) and high (≥2 or ≥1 severe exacerbation) risk categories [6]. The critical difference between these classification systems is that, according to the ECLIPSE classification, one hospitalization (severe exacerbation) would suggest an infrequent exacerbator, whereas it would suggest a high-risk exacerbator according to the new GOLD classification [6, 8].
Few studies have investigated the effect of pharmacotherapy on COPD exacerbations in different subsets of exacerbator phenotype patients. The Prevention of Exacerbations with Tiotropium in Chronic Obstructive Pulmonary Disease (POET-COPD®) (ClinicalTrials.gov NCT00563381) study was a 1-year, randomized, double-blind, double-dummy, parallel-group trial that compared the effect of treatment with the long-acting anticholinergic tiotropium (18 μg via the HandiHaler® device once daily) (SPIRIVA®, Boehringer Ingelheim Pharma and Co. KG, Ingelheim, Germany) with that of the long-acting β2-agonist (LABA) salmeterol (50 μg via a hydrofluoroalkane-pressurized metered-dose inhaler twice daily) on the incidence of moderate and severe exacerbations in 7376 patients with COPD and a history of exacerbations in the preceding year [9]. The POET-COPD trial showed that, among the total patient population, tiotropium as compared with salmeterol (1) increased the time to the first exacerbation, (2) significantly increased the time to the first severe exacerbation, and (3) reduced the annual number of moderate or severe exacerbations [9]. However, the impact of tiotropium and salmeterol specifically on patients at low or high risk of exacerbations has not been established.
The current study therefore investigated the effect of tiotropium and salmeterol on exacerbations in patients considered to be at a low or high risk for exacerbations (GOLD guidelines 2014 classification) and frequent/infrequent exacerbators (ECLIPSE study criteria) during a 1-year treatment period in a post hoc analysis of patients in the POET-COPD trial.
Methods
Study Design
POET-COPD was a 1-year, randomized, double-blind, double-dummy, parallel-group trial that analyzed the effect of tiotropium 18 μg once daily or salmeterol 50 μg twice daily on moderate to severe exacerbations in patients with COPD [9]. The primary endpoint of the trial was time to first moderate or severe exacerbation. Secondary and safety endpoints included time to event, number of events, serious adverse events, and mortality. An exacerbation was defined as an increase in, or new onset of, at least two respiratory symptoms (cough, sputum, dyspnea, wheezing, chest tightness) with at least one symptom lasting for ≥3 days and leading the patient’s attending physician to initiate treatment with systemic corticosteroids and/or antibiotics (moderate exacerbation) or request hospitalization (severe exacerbation) [8, 10]. Patients receiving fixed-dose combinations of LABAs and inhaled corticosteroids (ICS) switched to ICS monotherapy (on equal ICS dose) at the start of randomized treatment. All COPD medications, except for anticholinergics or LABAs, were permitted during the double-blind treatment phase. Inclusion criteria were age ≥40 years, smoking history ≥10 pack-years, diagnosis of COPD (post-bronchodilator forced expiratory volume in 1 s [FEV1] ≤70% predicted and FEV1/forced vital capacity ≤70%), and at least one exacerbation in the previous year requiring treatment with systemic steroids and/or antibiotics and/or hospitalization.
Statistical Analysis
The statistical tests were designed to test the hypothesis that tiotropium had a greater efficacy than salmeterol in preventing future exacerbations. Post hoc analyses were performed by the subgroups that were low/high risk at baseline and based on collected COPD characteristics, including hospitalization due to an exacerbation and medication use. A Cox proportional hazards regression model was used for time-to-exacerbation analyses. Hazard ratios (HR) and p values were based on stratified Cox regression with treatment as covariate. Poisson regression correcting for overdispersion and adjusting for treatment exposure and with terms for subgroup and treatment-by-subgroup interaction was used for number-of-event (exacerbations) analyses. The probability of a patient being considered at high risk for exacerbations during 1 year of treatment in the POET-COPD study was analyzed using a log-binomial model adjusting for treatment exposure and with terms for subgroup and treatment-by-subgroup interaction. A post hoc subgroup analysis was conducted to compare patients in each study group who were and were not receiving ICS at baseline. Statistical analyses were conducted using SAS® version 9.2, SAS Institute Inc. 2009, Cary, North Carolina, USA.
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Results
The baseline characteristics of patients stratified according to low/high exacerbation risk is summarized in Table 1. Except for baseline lung function and baseline ICS use, patients in the low-risk (n = 2610) and high-risk (n = 4284) groups were balanced. Within the risk subgroups, there were no clinically relevant differences in the baseline characteristics of patients in the tiotropium and salmeterol groups (data not shown).
Time to First COPD Exacerbation
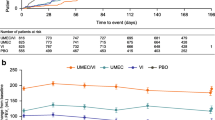
Using the GOLD 2013 risk classification for exacerbations, tiotropium reduced the risk of a COPD exacerbation compared with salmeterol in patients classified as being at high risk or low risk for exacerbations (Fig. 1). A similar pattern was obtained when using the frequent/infrequent risk classification definition. Tiotropium also increased the time to first COPD exacerbation compared with salmeterol in patients classified as being at high risk for exacerbations (HR 0.84; 95% confidence interval [CI] 0.76–0.92; p = 0.0002) (Table 2). In those patients classified as being at low risk for exacerbations, there was a non-significant increase in time to first COPD exacerbation for tiotropium compared with salmeterol (HR 0.89; 95% CI 0.77–1.02; p = 0.1046) (Table 2).
A similar pattern was obtained when using the frequent/infrequent risk classification definition. Tiotropium significantly increased the time to first COPD exacerbation compared with salmeterol in frequent exacerbators (HR 0.84; 95% CI 0.76–0.93; p = 0.0005) and infrequent exacerbators (HR 0.87; 95% CI 0.77–0.99; p = 0.0414) (Table 2). Whether or not patients were on background ICS at baseline did not impact these results (Table 3).
Number of COPD Exacerbations (Adjusted Annual Rates)
Tiotropium significantly reduced the number of COPD exacerbations compared with salmeterol in patients classified as being at high risk for exacerbations (rate ratio [RR] 0.90; 95% CI 0.81–0.99; p = 0.0383; adjusted annual rates = 0.76 and 0.85 for tiotropium and salmeterol, respectively) (Fig. 2). When using the frequent/infrequent risk classification, there was a borderline significant reduction with tiotropium in the number of COPD exacerbations compared with salmeterol in patients classified as frequent exacerbators (RR 0.90; 95% CI 0.81–1.01; p = 0.0668; adjusted annual rates = 0.79 and 0.87 for tiotropium and salmeterol, respectively) and also in the number of COPD exacerbations compared with salmeterol in patients classified as infrequent exacerbators (RR 0.88; 95% CI 0.75–1.02; p = 0.0881; adjusted annual rates = 0.48 and 0.55 for tiotropium and salmeterol, respectively) (Fig. 2). In patients classified as being at low risk for exacerbations, there was a borderline significant reduction with tiotropium in the number of COPD exacerbations compared with salmeterol (RR 0.89; 95% CI 0.76–1.05; p = 0.1768; adjusted annual rates = 0.48 and 0.54 for tiotropium and salmeterol, respectively) (Fig. 2). The post hoc subgroup analysis comparing both high- and low-risk patients who were or were not receiving ICS at baseline showed similar trends towards tiotropium reducing the number of COPD exacerbations; however, in high-risk patients receiving ICS at baseline, this was a numerical difference only (Fig. 3).
Treatment-Induced Shifts in Patient Risk Classification
Comparing patients at low versus high risk for exacerbations before and after 1 year’s treatment with either tiotropium or salmeterol showed that treatment resulted in a shift in the ratio (low/high exacerbation risk) from 0.62 to 4.98 and from 0.60 to 4.25 for treatment with tiotropium and salmeterol, respectively (Table 4). When using the infrequent versus frequent for exacerbations definition, shifts in the ratio (infrequent/frequent exacerbations) were larger (from 0.82 to 6.44 and from 0.78 to 5.80 for treatment with tiotropium and salmeterol, respectively) (Table 4). The risk of remaining in the high-risk subgroup for exacerbations after 1 year of treatment was statistically lower in the tiotropium group than in the salmeterol group (RR 0.89; 95% CI 0.80–1.00; p = 0.0478) (Table 5). The risk of patients who were in the low-risk subgroup for exacerbations in the previous year being considered at high risk for exacerbations after 1 year’s treatment was statistically lower in the tiotropium group compared with salmeterol (RR 0.87; 95% CI 0.71–1.07; p = 0.1968) (Table 5).
Discussion
This post hoc analysis of the POET-COPD trial, which investigated the effect of tiotropium and salmeterol on exacerbations in patients considered to be at a low or high risk for exacerbations during the 1-year treatment period, had two main findings. First, it confirmed the higher efficacy of tiotropium compared with salmeterol in prolonging the time to first COPD exacerbation and reducing the number of COPD exacerbations in patients considered to be at low risk, as well as in those considered to be at high risk, for exacerbations. Second, our results show that background ICS usage at baseline, or lack thereof, had no impact on tiotropium’s efficacy (versus salmeterol) with regard to reducing the exacerbation frequency among patients with COPD, regardless of their risk classification. Although the original trial was not set up to test this, tiotropium, and to a lesser extent salmeterol, appears to shift patients in the high-risk classification group to the low-risk group, suggesting a stabilizing effect on the disease.
The initial observation from the current analysis, that tiotropium had a greater efficacy than salmeterol in prolonging the time to first COPD exacerbation, confirms the primary results among the total patient population for the POET-COPD trial. Additionally, although not formally tested, our results suggest that the impact of tiotropium versus salmeterol on exacerbations was greater among those patients classified as being at high risk for exacerbations, regardless of whether the ECLIPSE [8] or recent GOLD [6] definitions are applied. In those patients classified as being at low risk for exacerbations, the results were borderline significant in favor of tiotropium, whereas in the high-risk group, this difference was statistically significant in favor of tiotropium. This might be explained by a lower number of exacerbations in the low-risk group.
It is noteworthy that the comparable effect observed from treatment with tiotropium versus salmeterol on COPD exacerbations does not appear to be influenced by the concomitant use of background ICS, although the risk ratio is more favorable for tiotropium when compared with salmeterol in both low- and high-risk exacerbator groups that were not using ICS.
Not only did our results show that treatment with tiotropium or salmeterol resulted in a higher proportion of patients shifting from the high-risk (frequent) to the low-risk (infrequent) exacerbator group, but also that treatment prevented patients at low risk of exacerbations from transitioning into the high-risk group. Many studies have shown that intervention with pharmacotherapy confers benefit to patients with COPD by reducing exacerbations or prolonging the time to first exacerbation [11–14]; however, few have successfully demonstrated that pharmacotherapy can positively influence the future risk of patients experiencing exacerbations [15]. As observed in other interventional trials both in COPD and asthma [13, 16], the overall shift in the exacerbation frequency prior to enrollment versus randomized treatment is remarkable and somehow surprising, particularly in comparison to the relative stability of the phenotype in an observational setting [17]. While insufficient background COPD treatment prior to inclusion may at least partially explain this strong shift, other factors may also be relevant such as the definition and documentation of exacerbations [18] (e.g., post hoc for history of exacerbations vs. prospectively on trials) as well as recall or reporting of exacerbations [19].
We speculate that by intervening early in the disease process, it may therefore be possible to minimize deleterious effects associated with increased frequency and severity of exacerbations in patients with COPD.
A limitation of the current study is that the POET-COPD trial was not designed to evaluate the effect of tiotropium specifically in different exacerbator phenotypes, nor was it designed to evaluate the effect of tiotropium on mitigating future risk for exacerbations. However, the detailed information regarding exacerbation history before enrollment and subsequently recorded over the course of the study, the large number of patients that participated, and the careful attention to study outcomes provides confidence in the results.
Conclusion
The results from the current study provide further evidence for intervention with tiotropium as maintenance therapy in COPD patients both at low or high risk for exacerbations.
References
Seemungal TA, Hurst JR, Wedzicha JA. Exacerbation rate, health status and mortality in COPD—a review of potential interventions. Int J Chron Obstruct Pulmon Dis. 2009;4:203–23.
Soler-Cataluna JJ, Martinez-Garcia MA, Roman SP, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–31.
Niewoehner DE. The impact of severe exacerbations on quality of life and the clinical course of chronic obstructive pulmonary disease. Am J Med. 2006;119(10 Suppl 1):38–45.
Anzueto A. Impact of exacerbations on COPD. Eur Respir Rev. 2010;19(116):113–8.
Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–54.
Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–65.
Hoogendoorn M, Feenstra TL, Hoogenveen RT, Al M, Molken MR. Association between lung function and exacerbation frequency in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2010;5:435–44.
Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–38.
Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364(12):1093–103.
Beeh KM, Glaab T, Stowasser S, et al. Characterisation of exacerbation risk and exacerbator phenotypes in the POET-COPD trial. Respir Res. 2013;14:116.
Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–89.
Dransfield MT, Bourbeau J, Jones PW, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med. 2013;1(3):210–23.
Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1(3):199–209.
White P. Inhaled fluticasone and budesonide increased the risk of serious pneumonia in COPD. Evid Based Med. 2014;19(3):116.
Wedzicha JA, Rabe KF, Martinez FJ, et al. Efficacy of roflumilast in the COPD frequent exacerbator phenotype. Chest. 2013;143(5):1302–11.
Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I, Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol. 2009;124(6):1210–6.
Donaldson GC, Mullerova H, Locantore N, et al. Factors associated with change in exacerbation frequency in COPD. Respir Res. 2013;14:79.
Mackay AJ, Donaldson GC, Patel AR, Singh R, Kowlessar B, Wedzicha JA. Detection and severity grading of COPD exacerbations using the exacerbations of chronic pulmonary disease tool (EXACT). Eur Respir J. 2014;43(3):735–44.
Langsetmo L, Platt RW, Ernst P, Bourbeau J. Underreporting exacerbation of chronic obstructive pulmonary disease in a longitudinal cohort. Am J Respir Crit Care Med. 2008;177(4):396–401.
Acknowledgments
Sponsorship, article processing charges, and the open access charge for this study were funded by Boehringer Ingelheim and Pfizer. The POET-COPD trial was funded by Boehringer Ingelheim and Pfizer.
The authors acknowledge medical writing and editorial assistance from Godfrey Lisk, PhD, Senior Scientific Specialist at PAREXEL, in the form of generation of figures and tables and preparation and revision of the draft manuscript, which was funded by Boehringer Ingelheim and Pfizer. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Conflict of interest
Claus F. Vogelmeier gave presentations at symposia and/or served on scientific advisory boards sponsored by Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Grifols, Janssen, Mundipharma, Novartis, and Takeda. He was responsible for planning and interpretation of data, as well as drafting the manuscript.
The institution where Kai M. Beeh is employed has received compensation for organizing or participating in advisory boards for Almirall Hermal, AstraZeneca, Boehringer Ingelheim, Chiesi, Cytos, Mundipharma, Novartis, and Revotar Biopharmaceuticals and for participation in scientific meetings or courses supported by various pharmaceutical companies (Almirall Hermal, AstraZeneca, Boehringer Ingelheim, Novartis, Pfizer, and Takeda) in the past 3 years. His institution has also received consulting fees from Ablynx, Apellis Pharmaceuticals, Chiesi, and Cytos and compensation for the design, performance, or participation in single- or multicenter clinical trials in the past 3 years from several companies, including Almirall, Boehringer Ingelheim, Cytos, GlaxoSmithKline, Mundipharma, Novartis, Pfizer, Revotar Biopharmaceuticals, Sterna AG, and TEVA. He was responsible for planning and interpretation of data, as well as drafting the manuscript. Guus M. Asijee is an employee of Boehringer Ingelheim and was responsible for planning and interpretation of data, as well as drafting the manuscript. Katrin Kupas is an independent statistician hired by Boehringer Ingelheim and was responsible for statistical analysis, interpretation of data, and drafting the manuscript.
Guarantor author
Guus M. Asijee, PhD.
Contributors
We thank the patients who participated in the trial; Dr. Klaus Fichtner, Vera Drews, and Nicole Bader for administrative trial support; Dr. Steven Kesten, Dr. Fee Rühmkorf, Dr. Harald Koegler, Dr. Susanne Stowasser, Dr. Brigitta Monz, and Dagmar Selim for scientific advice; Dr. Inge Leimer and Achim Müller for statistical advice; Michael Betke-Hornfeck for technical trial support; and Christine Meissner and Christina Raabe for data management support.
Compliance with ethics guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Vogelmeier, C.F., Asijee, G.M., Kupas, K. et al. Tiotropium and Salmeterol in COPD Patients at Risk of Exacerbations: A Post Hoc Analysis from POET-COPD® . Adv Ther 32, 537–547 (2015). https://doi.org/10.1007/s12325-015-0216-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-015-0216-2