Abstract
Muscle weakness is one of the most serious problems during chemotherapy for childhood hematological malignancies. It may be caused by long-term hospitalization, unfavorable physical conditions, and restricted activities. Although the concept of sarcopenia is becoming widely recognized, especially in geriatric medicine, there have been few reports about sarcopenia in pediatric patients with hematological malignancies. A total of 47 consecutive first-onset acute lymphoblastic leukemia (ALL) patients who underwent induction therapy between January 2011 and September 2016 were investigated. The cross-sectional psoas muscle area (PMA) was measured on computed tomography (CT) images. PMA changes were expressed as the Muscle Loss Index (MLI), which was calculated by dividing the post-treatment PMA by the pre-treatment PMA. In this study, patients with values less than the lowest quartile of MLI were classified into the sarcopenia group, and their basic and clinical factors were compared with those in the non-sarcopenia group. Muscle loss was observed in all patients after induction therapy, and severe adverse events during induction therapy were significantly more common in patients in the sarcopenia group. Furthermore, sarcopenia was found to be an independent prognostic factor for invasive fungal infection (IFI) that occurs after induction therapy. The evaluation of sarcopenia on CT images is easy and useful as a predictor of unfavorable events such as IFI in the treatment of childhood ALL.
Similar content being viewed by others
Introduction
Sarcopenia is a common disorder among elderly people. It is reported to be associated with complications after surgery in adult patients. However, there have been few reports on the clinical importance of sarcopenia in children. On the other hand, muscle weakness is a critical problem frequently observed in the treatment of childhood hematological malignancies. In this study, the focus was on the change in skeletal muscle volume following induction therapy in children with acute lymphoblastic leukemia (ALL). The aim was to identify how often sarcopenia appears and how it affects the patients’ clinical courses during chemotherapy.
Methods
Patients
The medical records of 47 consecutive first-onset pediatric ALL patients who underwent induction therapy at our hospital between January 2011 and September 2016 were retrospectively reviewed. Those who previously underwent any chemotherapy were excluded. This study was approved by the Ethics Committee of our hospital. We obtained informed consent from all cases.
Background data
For all enrolled patients, the following demographic and clinical data were evaluated as background factors related to sarcopenia: sex, age, risk of disease (standard risk or high risk, as defined by the National Cancer Institute), and adverse events observed during induction therapy.
Clinical data and image analysis
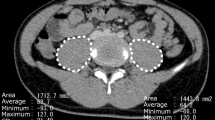
To evaluate the changes in patients’ muscle volume from before to after the induction therapy, the sum of the cross-sectional areas of the bilateral psoas major muscles was used. The psoas muscle area (PMA) was calculated on CT images at the level of the L3 vertebra. Calculation of PMA was performed using the picture archiving and communication system EV insite® version 3.1.200.32 (PSP Corporation, Tokyo, Japan).
Definition and assessment of sarcopenia
To clarify the severity of muscle loss, PMA changes were calculated by dividing the post-treatment PMA by the pre-treatment PMA to obtain the Muscle Loss Index (MLI). In this study, the measurement interval between pre- and post-treatment was roughly 35 days, and patients with values less than the lowest quartile of MLI were classified into the sarcopenia group.
Clinical outcomes
Mortality and infectious complications were evaluated as clinical outcomes and compared between the two groups.
Statistical analysis
Student’s t test, the χ 2-test, and the Mann–Whitney U test were used to evaluate the changes of patients’ status. Multivariate stepwise regression analysis was used to determine the independence of variables. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R Commander designed to add statistical functions frequently used in biostatistics.
Results
Of the 47 patients, 24 were boys and 23 were girls. The age of patients at onset ranged from 0.9 to 16 years (median 8.6 years). Forty-two patients had B precursor lymphoblastic leukemia, 4 had T-cell lymphoblastic leukemia, and 1 had mature B cell lymphoblastic leukemia. The induction therapy included steroids (prednisolone, dexamethasone), vincristine, cyclophosphamide, THP-adriamycin, daunomycin, l-asparaginase, methotrexate, and cytarabine. During induction therapy, adverse events of grade III or more, defined using Common Terminology Criteria for Adverse Events (CTCAE) version 4.03, occurred in 11 patients. The adverse events included intracranial hemorrhage in 3 patients, acute kidney injury in 5 patients, seizure in 1 patient, bone pain in 1 patient, and vomiting in 1 patient.
Median body weight decreased significantly from 31.2 (range 7.9–72.8) to 25.8 kg (range 8.6–59.7 kg). However, to be more precise, 32 patients lost weight, while 15 gained weight after induction therapy. On the other hand, PMA decreased in all patients, from 1162.8 (range 324.6–3699.8) to 810.6 mm2 (range 290.8–2709.2 mm2) (Figure 1). The median MLI was 0.74, ranging from 0.41 to 0.99. In this study, 14 of 47 patients had MLI less than 0.67, which corresponded to the lowest quartile of MLI, and they were classified into the sarcopenia group.
Regarding the background factors for sarcopenia, age, sex, and disease risk were not related to sarcopenia, but patients who had adverse events of grade III or more during induction therapy were significantly more likely to have sarcopenia (P = 0.009) (Table 1).
In this study, seven out of 47 patients develop invasive fungal infection (IFI) after the induction therapy. As clinical outcomes potentially related to sarcopenia, mortality and infectious complications such as bacteremia and IFI were evaluated. In this study, sarcopenia was not significantly related to mortality, while it was related to IFI (Table 1).
Discussion
Sarcopenia was first described by Rosenberg [1] as a disorder characterized by progressive and generalized loss of skeletal muscle mass and strength that can cause physical disability and poor quality of life. Although it is widely recognized as part of a geriatric syndrome called “age-related sarcopenia”, there are also other forms of sarcopenia that are related to activity range, disease, and nutrition. Sarcopenia is considered a useful predictor for poor outcomes after chemotherapy, surgery, and trauma in adult patients [2, 3]. However, there have been few reports about sarcopenia in children. Rayar et al. reported the incidence of sarcopenia in children with acute lymphoblastic leukemia; a notable reduction in skeletal muscle mass occurred early in the treatment and was associated with the duration of hospitalization [4].
The present retrospective study demonstrated that muscle loss was observed in all patients after induction therapy, and sarcopenia was significantly associated with severe adverse events during induction therapy. Furthermore, sarcopenia was found to be an independent prognostic factor for IFI. This was unexpected. This report shows the clinical impact of sarcopenia in childhood ALL. We previously reported the risk factors for IFI in pediatric hematologic and malignant diseases to be acute myeloid leukemia, age ≥ 10 years, and relapse of original disease [5], and based on the finding of the present study, sarcopenia can be seen as another risk factor for IFI. There has also been a report on the association between sarcopenia and infectious complications; Takagi et al. reported sarcopenia as a predictor for postoperative infectious complications. They speculated that decreased interleukin-15 due to the depletion of skeletal muscle and subsequent synthesis of proinflammatory adipokines from increased adipose tissue might be associated with the interaction between sarcopenia and immune depression [6].
Functional muscle status in terms of handgrip strength, gait speed, and exhaustion was not evaluated in the present study. There are already some criteria of sarcopenia published by groups from Europe [7], the USA [8] and Asia [9]. In these criteria, muscle mass is supposed to be measured by dual energy X-ray absorptiometry or bioimpedance analysis, and muscle function should be evaluated by handgrip strength and gait speed. However, these criteria cannot be applied to children, because pediatric patients consist of infants, toddlers, and young adults.
In this context, radiographic evaluation of skeletal muscle volume is comparatively easy, even in pediatric patients. There already have been some papers about evaluating sarcopenia using PMA at the L3 vertebra on CT images, and its usefulness and simplicity are becoming widely recognized [10,11,12]. In the present study, the focus was on the change of PMA during chemotherapy, and regarding the definition of sarcopenia, a simple and comprehensive indicator, the MLI, was established to evaluate the degree of muscle reduction. We considered that this method would more precisely evaluate skeletal muscle reduction in patients of different ages and physical frames. On the basis of these results, early nutritional intervention and physical treatment can be planned properly.
Despite the important findings in this study, several limitations should be discussed. First, this was a small, single-center, retrospective study. Second, the MLI was used to assess muscle reduction, and sarcopenia was defined as an MLI less than the 25th‰. Further studies are needed to identify the precise cut-off value for sarcopenia for predicting clinical complications. Finally, the interaction between sarcopenia and IFI has yet to be fully explained. Further analyses are needed to identify additional factors related to IFI.
Conclusion
Evaluation of sarcopenia in the treatment of childhood ALL is easy and practical. We should pay more attention to sarcopenia to improve the patients’ prognosis, as well as their quality of life.
Abbreviations
- ALL:
-
Acute lymphoblastic leukemia
- PMA:
-
Psoas major muscle area
- CT:
-
Computed tomography
- MLI:
-
Muscle Loss Index
- IFI:
-
Invasive fungal infection
- NCI:
-
National cancer institute
- CTCAE:
-
Common terminology criteria for adverse events
References
Rosenberg I. Summary comments. Am J Clin Nutr. 1989;50:1231–3.
Hara N, Iwasa M, Sugimoto R, Mifuji-Morooka R, Yoshikawa K, Terasawa E, et al. Sarcopenia and sarcopenic obesity are prognostic factors for overall survival in patients with cirrhosis. Intern Med. 2016;55:863–70.
Begini P, Gigante E, Atonelli G, Carbonetti F, Iannicelli E, Anania G, et al. Sarcopenia predicts reduced survival in patients with hepatocellular carcinoma at first diagnosis. Ann Hepatol. 2017;16:107–14.
Rayar M, Webber CE, Nayiager T, Sala A, Barr RD. Sarcopenia in children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2013;35:98–102.
Kobayashi R, Kaneda M, Sato T, Ichikawa M, Suzuki D, Ariga T. The clinical feature of invasive fungal infection in pediatric patients with hematologic and malignant diseases: a 10-year analysis at a single institution at Japan. J Pediatr Hematol Oncol. 2008;30:886–90.
Takagi K, Yoshida R, Yagi T, Umeda Y, Nobuoka D, Kuise T, et al. Radiographic sarcopenia predicts postoperative infectious complications in patients undergoing pancreaticoduodenectomy. BMC Surg. 2017;17:64.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Ageing. 2010;39:412–23.
Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–56.
Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101.
Hanaoka M, Yasuno M, Ishiguro M, Yamauchi S, Kikuchi A, Tokura M, et al. Morphologic change of the psoas muscle as a surrogate marker of sarcopenia and predictor of complications after colorectal cancer surgery. Int J Colorectal Dis. 2017;32:847–56.
Ikeno Y, Koide Y, Abe N, Matsueda T, Izawa N, Yamazato T, Matsumori M, Tanaka H, Ishihara S, Nakayama S, Sugimoto K, Okita Y, et al. Impact of sarcopenia on the outcomes of elective total arch replacement in the elderly. Eur J Cardiothorac Surg. 2017;51:1135–41.
Harada K, Suzuki S, Ishii H, Aoki T, Hirayama K, Shibata Y, et al. Impact of skeletal muscle mass on long-term adverse cardiovascular outcomes in patients with chronic kidney disease. Am J Cardiol. 2017;119:1275–80.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Suzuki, D., Kobayashi, R., Sano, H. et al. Sarcopenia after induction therapy in childhood acute lymphoblastic leukemia: its clinical significance. Int J Hematol 107, 486–489 (2018). https://doi.org/10.1007/s12185-017-2388-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-017-2388-9