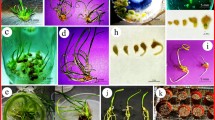
Abstract
An efficient protocol for plant regeneration was developed from protoplasts of Gentiana macrophylla Pall. through somatic embryogenesis. Viable protoplasts were isolated from cell suspensions derived from young seedling leaves in an enzyme solution containing 2 % Cellulase Onozuka R-10, 0.5 % Macerozyme R-10, 0.5 % Hemicellulase, and 0.4 M sorbitol with a yield of 6.2 × 106 protoplasts g−1 fresh weight. Liquid, solid–liquid double layer (sLD) and agar-pool (aPL) culture systems were used for protoplast culture. The frequency of protoplast cell divisions and colony formations in aPL culture were significant (p < 0.05) higher than those in liquid and sLD cultures. The highest division frequency and plating efficiency were 37.7 % and 16.5 %, respectively, in aPL culture supplemented with 2 mg l−1 2,4-dichlorophenoxyacetic acid (2,4-d) and 0.5 mg l−1 6-benzylaminopurine (BA). Protoplast-derived microcalli obtained from aPL culture system were transferred to solid MS medium with a reduced concentration of 2,4-d (0.5 mg l−1) to promote the formation of embryogenic calli. Somatic embryos developed into plantlets on MS medium supplemented with 2 mg l−1 BA at a rate of 51.3 %. RAPD analysis of G. macrophylla revealed a low variation among regenerants.



Similar content being viewed by others
Abbreviations
- aPL:
-
Agar-pool culture
- BA:
-
6-Benzylaminopurine
- CH:
-
Casein hydrolysate
- 2,4-d :
-
2,4-Dichlorophenoxyacetic acid
- FDA:
-
Fluorescein diacetate
- MES:
-
2-(N-morpholino) ethanesulfonic acid
- MS:
-
Murashige and Skoog (1962) medium
- sLD:
-
Solid-liquid double layer culture
- RAPD:
-
Random amplified polymorphic DNA
References
Bai Y, Han N, Wu JX, Yang YN, Wang JH, Zhu MY, Bian HW (2014) A transient gene expression system using barley protoplasts to evaluate microRNAs for post-transcriptional regulation of their target genes. Plant Cell Tissue Organ Cult 119:211–219
Cao JP, Liu X, Hao JG, Zhang XQ (2005) Tissue culture and plantlet regeneration of Gentiana macrophylla. Acta Bot Boreal Occident Sin 25:1101–1106
Chen LP, Zhang MF, Xiao QB, Wu JG, Hirata Y (2004) Plant regeneration from hypocotyl protoplasts of red cabbage (Brassica oleracea) by using nurse cultures. Plant Cell Tissue Organ Cult 77:133–138
Chen LY, Chen QL, Xu D, Hao JG, Michael S, Xu ZQ (2009) Changes of gentiopicroside synthesis during somatic embryogenesis in Gentiana macrophylla. Planta Med 75:1618–1624
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Du L, Bao MZ (2005) Plant regeneration from protoplasts isolated from embryogenic suspension cultured cells of Cinnamomum camphora L. Plant Cell Rep 24:462–467
Fiuk A, Rybczyński JJ (2007) The effect of several factors on somatic embryogenesis and plant regeneration in protoplast cultures of Gentiana kurroo (Royle). Plant Cell Tissue Organ Cult 91:263–271
Harter HL (1960) Critical values for Duncan’s multiple range test. Biometrics 16:671–685
Jiang ZB, Liu HL, Liu XQ, Shang JN, Zhao JR, Yuan CS (2010) Chemical constituents of Gentiana macrophylla Pall. Nat Prod Res 24:1365–1369
Jomori H, Takahata Y, Kaizuma N (1995) Plant regeneration from leaf-derived calli of gentians and their protoplast culture. Acta Hort 392:81–86
Ma R, Guo YD, Pulli S (2003) Somatic embryogenesis and fertile green plant regeneration from suspension cell-derived protoplasts of rye (Secale cereale L.). Plant Cell Rep 22:320–327
Meng Y, Gao Y, Jia J (1996) Plant regeneration from protoplasts isolated from callus of Gentiana crassicaulis. Plant Cell Rep 16:88–91
Mikula A, Tomiczak K, Rybczyński JJ (2011) Cryopreservation enhances embryogenic capacity of Gentiana cruciata (L.) suspension culture and maintains (epi) genetic uniformity of regenerants. Plant Cell Rep 30:565–574
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissues cultures. Physiol Plant 15:473–497
Nakano M, Hosokawa K, Oomiya T, Yamamura S (1995) Plant regeneration from protoplasts of Gentiana by embedding protoplasts in gellan gum. Plant Cell Tissue Organ Cult 41:221–227
Qi XJ, Chen RY, Wang W (2010) Cell suspension culture of Gentiana macrophylla. Chin Tradit Herb Drug 41:636–638
Takahata Y, Jomori H (1989) Plant regeneration from mesophyll protoplasts of Gentiana (Gentiana scabra Bunge). Plant Tissue Cult Lett 6:19–21
Tang J, Yang Y, Li M, Xu Q, Wang J (2006) Research of botanical resources and utilization of Gentiana macrophylla in Liupan Mountain. J Agric Sci 27:59–62
Tiwari RK, Trivedi M, Guang ZC, Guo GQ, Zheng GC (2007) Genetic transformation of Gentiana macrophylla with Agrobacterium rhizogenes: growth and production of secoiridoid glucoside gentiopicroside in transformed hairy root cultures. Plant Cell Rep 26:199–210
Wang J, Jiang JJ, Wang YP (2013) Protoplast fusion for crop improvement and breeding in China. Plant Cell Tissue Organ Cult 112:131–142
Widholm J (1972) The use of FDA and phenosafranine for determining viability of cultured plant cells. Stain Technol 47:186–194
Wu HJ, Wang XX, Li Y, Zhang DG, Zhang B, Wang XY (2011) Propagation of Gentiana macrophylla (Pall) from hairy root explant via indirect somatic embryogenesis and gentiopicroside content in obtained plants. Acta Physiol Plant 33:2229–2237
Xiao W, Huang XL, Huang X, Chen YP, Dai XM, Zhao JT (2007) Plant regeneration from protoplasts of Musa acuminate cv. Mas (AA) via somatic embryogenesis. Plant Cell Tissue Organ Cult 90:191–200
Yang X, Guo X, Zhang X, Nie Y, Jin S (2007) Plant regeneration from Gossypium davidsonii protoplasts via somatic embryogenesis. Biol Plant 51:533–537
Zhang HL, Xue SH, Pu F, Tiwari RK, Wang XY (2010) Establishment of a hairy root line and analysis of its gentiopicroside compound in medicinal plant Gentiana macrophylla Pall. Russ J Plant Physiol 57:117–124
Acknowledgments
We thank the anonymous referees for their helpful comments on the manuscript. This work was financially supported by the National Natural Science Foundation of China (30960161, 31360095).
Author information
Authors and Affiliations
Corresponding author
Additional information
Xuemin Hu and Yao Yin have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hu, X., Yin, Y. & He, T. Plant regeneration from protoplasts of Gentiana macrophylla Pall. using agar-pool culture. Plant Cell Tiss Organ Cult 121, 345–351 (2015). https://doi.org/10.1007/s11240-014-0705-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-014-0705-z