Abstract
Background and aims
The Serengeti grassland is characterized by antiparallel gradients of soil phosphorus (P) and precipitation. We hypothesized that grasses associate with arbuscular mycorrhizal (AM) fungi to ameliorate water stress and improve nutrient acquisition; and, that geographic patterns in AM fungal abundance relate to nutrient and water limitation of host plants.
Methods
We conducted a factorial experiment to uncouple the interacting effects of soil type, P, and water availability on AM fungal abundance. Maize was grown in pots of soil collected from three locations across the natural gradients. Full factorial treatments of +/− P fertilization and high/low water were administered to all three soils.
Results
Abundance of AM hyphae in soil was reduced by fertilization in high-P soil and increased with fertilization in low-P soil. Phosphorus uptake efficiency of mycorrhizas was greatest in low-P soil. Water-limited plants grown in arid region soil allocated relatively more biomass to AM fungi.
Conclusions
The formation of AM fungi in each soil was most strongly linked to the most limiting belowground resource. Interactions among soil properties, water availability and variation in the community composition of AM fungi are likely to influence the abundance and function of AM symbioses the Serengeti.
Similar content being viewed by others
Introduction
Most grassland plants form symbiotic relationships with arbuscular mycorrhizal (AM) fungi (Miller et al. 2012). These fungi, in the phylum Glomeromycota, exchange mineral resources for plant photosynthate. Hyphae of AM fungi grow into the soil beyond the rhizosphere of the host plant to acquire primarily phosphorus (P; Bolan 1991), but also nitrogen (N; George et al. 1995) and various micronutrients (Marschner and Dell 1994; Al Karaki and Clark 1998). The symbiosis also influences plant water relations and drought resistance (Augé 2001; Augé et al. 2014). Although AM associations are generally mutually beneficial for plants and fungi, mycorrhizal function varies from mutualism to parasitism (Johnson et al. 1997; Klironomos 2003). Resource availability determines the costs and benefits of mycorrhizas and influences mycorrhizal function (Johnson et al. 1997); P nutrition is often correlated with mycorrhizal function and the abundance of AM fungi (Mosse et al. 1973; Koide 1991; Miller et al. 1995). When plants are P limited, mycorrhizal benefits can be great, but when P is in ample supply the carbon (C) costs of the symbiosis may exceed the P benefits and depress plant growth (Bethlenfalvay et al. 1983; Peng et al. 1993). The Functional Equilibrium model proposed by Brouwer (1983) predicts plants will preferentially allocate biomass to the organs needed to acquire the most limiting resources. This hypothesis has been expanded to include allocation to AM fungi (Johnson et al. 2003, 2010). Field studies in North America (Johnson et al. 2003), the Tibetan plateau (Liu et al. 2012), and Germany (Blanke et al. 2011) provide empirical support for this model by showing that the abundance of AM fungi inside roots and in the soil is often reduced when plants are not limited by either P or N.
The Serengeti ecosystem is a marvel among a dwindling number of intact grassland ecosystems. It is prized for its vastness and its intact migrations of ungulates and other macrofauna. Geologic processes influence climate and soils and control the distribution of plant and animal communities of the Greater Serengeti Ecosystem. The rainshadow of the adjacent Ngorongoro highlands cause the south and east to be drier than the north and west (Fig. 1). Superimposed on this precipitation gradient is a fertility gradient resulting from deposition of volcanic ash (McNaughton 1985). These antiparallel gradients affect soil properties (Anderson and Talbot 1965), vegetation (McNaughton 1983; Anderson et al. 2007), microbial biomass, rates of C and N cycling (Ruess and Seagle 1994), and ultimately drive ungulate migration (McNaughton 1990; Frank et al. 1998).
Map of the precipitation and P gradients in the Serengeti National Park, Tanzania, (B. Stevens unpublished). Soil was collected from Kuku Hills (KUH), Seronera (SER) Soit Le Motonyi (SOT). Darker regions of the map indicate greater annual precipitation; darker site markers indicate higher total soil P. Numbers refer to the average total soil P (%) at each site
Weathering processes remove available forms of P from soil (Walker and Syers 1976); consequently, plants in highly weathered tropical soils are frequently severely P limited (Vitousek et al. 2010). Intermittent deposition of volcanic ash in the southern and eastern parts of Serengeti, combined with higher weathering rates caused by greater rainfall in the north and west have generated a natural gradient of soil P and provides a natural experiment to examine the relationship between soil fertility and the abundance of AM fungi. A study of AM fungi in soil collected from eight locations across this P gradient indicated that the density of AM hyphae in soil was positively correlated with total soil P (Johnson et al. 2013; Antoninka et al. in preparation). The direction of the observed correlation was opposite the pattern predicted by the Functional Equilibrium model: density of AM fungi was lowest in the P-depleted soils from the north and highest in the southern soils with higher P. The pattern was the same for soils collected during the wet season (April) as well as during the dry season (September), so it is unlikely that it was an artifact caused by different seasonal patterns of AM fungi in the northern and southern portions of the Serengeti.
There are several possible reasons for these unexpected findings, and not all are mutually exclusive (Table 1). Annual rainfall is inversely related to soil P in the Serengeti: dry sites have higher soil P compared to wetter sites. Host plants invest in AM fungi in response to limiting belowground resources; perhaps the AM fungal abundance is increasing in response to plant water limitation (H1). Arbuscular mycorrhizas are known to ameliorate plant water stress (Wu and Xia 2006; Augé et al. 2014). Hyphae of AM fungi increase physical contact with soil particles and reduce lethal matric potential (water potential of soil) for host plants (Augé 2004). The exchange of fungal-acquired P for plant photosynthate is understood as a highly regulated two-way exchange (Hammer et al. 2011; Fellbaum et al. 2012), but direct exchange of fungal-acquired water for photosynthate is unlikely. The mechanism driving mycorrhizal responses to a rainfall gradient is complex because water availability influences both C demand of the fungus and C supply by the host. If C limitation induced by water stress is severe, then plants may be expected to reduce their investment in AM symbioses.
Another possible explanation for the strong positive correlation between soil P and the abundance of external AM hyphae is that availability of soil P in the Serengeti is so low that AM fungal growth is P limited, and accordingly, more P in the southern sites can support higher densities of AM fungi than the wetter, northern sites with less P (H2). Other studies in severely P depleted soil indicate that AM fungal growth is P limited and P fertilizer amendments may actually enhance fungal growth (Sanginga et al. 1996; Cuenca et al. 2003). Treseder and Allen (2002) proposed a conceptual model that illustrates mycorrhizal abundance across a gradient of P or N availability (Fig. 2a). When soil fertility is extremely low, both mycorrhizal fungi and host plants will be nutrient limited. As soil fertility increases, nutrient limitation of fungal growth is ameliorated and fungal abundance will increase. However, when soil nutrients are in luxury supply for both fungi and their hosts, plants will reduce allocation of photosynthate to belowground organs (as predicted by the Functional Equilibrium model) and this will reduce the abundance of mycorrhizal fungi. Optimal growth of AM fungi is predicted when soil fertility is sufficient to support the fungus but limiting to the host plant, and thereby stimulating C allocation belowground. Soils in the northern Serengeti could be so P limited that growth of AM fungi is restricted compared to sites in the southern Serengeti. This could account for the pattern observed by Johnson et al. 2013 and Antoninka et al. (in preparation).
(a) Conceptual model of mycorrhizal responses to soil fertility proposed by Treseder and Allen (2002). The shaded region indicates an optimal mutualism where soil nutrition does not limit AM fungal growth, yet host plants are P limited and allocate C belowground. (b) If AM fungi are limited by P at Kuku Hills (KUH) and not limited by P at Soit Le Motonyi (SOT), then we predict that P fertilization should increase AM fungal abundance in maize grown in Kuku Hills (KUH) soil and decrease AM fungal abundance in maize grown in soil from Le Motonyi (SOT). The response should be intermediate for maize grown in soil from Seronera (SER). Figure modified from Treseder and Allen (2002)
Thirdly, differences in soil pH along the P gradient could generate the patterns of external AM hyphal abundance observed in the Serengeti (H3). Soil pH is generally basic in the dry southern sites compared to more acidic soil in the wetter northern sites (Anderson and Talbot 1965; Ruess and McNaughton 1987); and this soil pH gradient may influence external AM hyphae. Previous studies have shown the abundance of AM fungi increases linearly with increasing soil pH in gradients that extend from acidic towards a neutral pH (Anderson et al. 1984; Johnson et al. 1991; Coughlan et al. 2000). Few studies have examined AM fungal patterns in gradients that range from neutral to alkaline soil pH. If AM fungi are P limited and their responses to soil pH are linked to P availability, then we would expect their abundance to be greater in neutral soil pH than in either acidic or alkaline soils because this reflects the solubility of P ions in the soil solution (Brady and Weil 1996).
A final hypothesis to account for the observed pattern of hyphal abundance in soils along the antiparallel P-precipitation gradient is that AM fungi decompose faster in wet soil compared to dry soil. Consequently, a smaller standing crop of AM hyphae will be maintained in sites with high rainfall compared to sites with less rainfall (H4). Varying results of laboratory studies indicate that rates of AM hyphal turnover range from a few hours or weeks (Staddon et al. 2003; Fitter et al. 2004) to several months (Steinberg and Rillig 2003; Olsson and Johnson 2005). Studies in natural ecosystems have also reported both rapid and relatively slow AM hyphal turnover rates (Hernandez and Allen 2013; Treseder et al. 2010, respectively). Soil moisture may be an important determinant of hyphal turnover rates and consequently, the standing crop of hyphae in the soil.
The intention of the present study is to better understand the mechanisms that generate the observed pattern of increasing AM hyphal lengths in soils from dry sites with relatively greater soil P. Our factorial experiment was conducted in the Serengeti to uncouple the effects of water and P availability on the growth of AM fungi in association with maize (Zea mays L.), a domesticated tropical grass. Maize provides an excellent experimental host plant because of its uniform and rapid growth. The experiment examined the direct and interactive effects of ambient soil conditions, P, and water availability on the abundance and function of AM fungi to test the predictions of the first three hypotheses in Table 1.
Materials and methods
Experimental design
Responses of maize and associated AM fungi to soil type, P, and water availability were studied in a full factorial experiment conducted at the Tanzania Wildlife Research Institute research station, 5 km east of the village of Seronera in the Serengeti National Park. The experiment was composed of three soil types x two P treatments (+/− rock phosphate) x two water treatments (ample and stressed), replicated eight times for a total of 96 pots. Soils were known to vary in pH facilitating the test of pH effects on AM fungi. However, pH was not manipulated as part of the factorial design.
In May 2012, soil was collected from three sites: Kuku Hills (KUH) in the north where precipitation is relatively high and soil is relatively P deficient, Soit Le Motonyi (SOT) in the southeast which has less precipitation and relatively P rich soil, and Seronera (SER) which is located between these points and has intermediate levels of precipitation and total soil P (Fig. 1; Table 2). Soil was collected every 5 m along a 35 m transect, homogenized and used to fill 32 plastic pots (10 cm diameter x 15 cm height). Half of the pots from each soil type were enriched with rock phosphate (PO4 −3) fertilizer (Minjingu Mines & Fertilisers Ltd., Arusha, TZ) at a rate of 550 μg g−1 of soil. Minjingu soft rock PO4 −3 contains approximately 13.3 % P (Msolla et al. 2005). Consequently, fertilized pots were enriched with 75.6 μg P g−1 soil. Half of the fertilized and unfertilized pots were then assigned to receive ample water or an insufficient amount of water. Because the bottom of the pots allowed excess water to drain, the 12 treatments were isolated from each other using plastic barriers between treatments. This prevented cross-contamination of soils and AM fungi due to runoff and reabsorption through the bottom of the pots, but precluded randomization of the pots across rows. All 96 pots were placed outside and kept in a 1.5 m x 4.5 m steel cage that was carefully reinforced to protect the plants from baboons and other wildlife.
Pesticide-free seeds of a local landrace of maize were surface sterilized in 10 % ethanol for 3 min, rinsed in deionized water and soaked over-night. Seeds were germinated in wet towels and one sprouting seed was planted in each pot. Ample water was applied for the first week, and seedlings that did not successfully develop were replaced with seedlings grown in the same soil. During the following month, plants in the dry treatment received 15.5 ml water every 2 days, and those in the wet treatment received 36.5 ml every 2 days. The volume of water applied to the dry treatment was determined as the amount of water needed to keep the plants alive, but impose obvious symptoms of water stress and slow relative growth rate compared to well-watered treatments. During the first week of plant establishment, periodic rainfall was allowed to fall on all of the pots, but during subsequent weeks, all pots were covered with clear plastic during rains. Rains became increasingly infrequent from May to June.
Plant and mycorrhizal measurements
After 5 weeks, maize shoots were cut from the roots and dried in a solar oven for at least 3 days. Roots from each pot were removed from soil and washed. Approximately 0.5 g of fresh roots were randomly collected from each plant for mycorrhizal measurements and remaining roots were dried and weighed. Root subsamples were cleared with 5 % KOH and stained using the ink and vinegar method (Vierheilig et al. 1998). Mycorrhizal fungal structures within the root cortex (vesicles, arbuscules, coils, intraradical hyphae) along with other (non-AM) fungi were quantified using the magnified line intersect method of McGonigle et al. (1990). Soil from each pot was collected and homogenized. A 500 g subsample was dried for nutrient analysis, and another, separate subsample (approximately 100 g) was taken from the volume of homogenized pot soil and stored frozen for subsequent analysis of external AM hyphae.
External fungal hyphae were extracted from soil using the procedure of Rillig et al. (1999). Approximately 4 g of soil was suspended in a solution of sodium hexametaphosphate (3.75 mg L−1), and 2 ml of the suspension was transferred to 0.45 μm Millipore filter (Millipore Corp. Billerica, MA, USA) via vacuum pump. The material on the filter was stained for 5 min with 0.03 % trypan blue. The membrane filter was transferred to a slide and mounted in PermountTM. Slides were examined using a compound microscope at 200x. The length of fungal hyphae was quantified using a gridded reticule (Abbott et al. 1984) distinguishing between AM hyphae and other fungi using well-known morphological criteria (Friese and Allen 1991; Jakobsen et al. 1992). The hyphae from AM fungi were counted as two diameter size classes (>2.5 μm and < 2.5 μm) and by whether they were stained or unstained. The efficiency of hyphal extraction (efficiency coefficient) was determined for each soil type by repetitively re-suspending and re-extracting hyphae from an individual soil sample (Miller et al. 1995).
As a means to relate the amount of plant-derived C allocated to AM fungi relative to plant biomass, the proportion of fungal C per unit plant C was calculated. This was used as a coarse comparison of plant investment in mycorrhizal symbioses across treatments. External hyphal length measurements and average hyphal diameter were used to calculate biovolume, and biovolume was converted to biomass based on hyphal relative density of 1.3 g cm−3 and dry matter content of 0.25 g per cm−3 (Miller et al. 1995). Biomass C was determined from known elemental composition of maize shoots (43.6 %; Latshaw and Miller 1924), roots (42.8 %; Amos and Walters 2006) and AM fungi (42 %; R. M. Miller, personal communication).
Nutrient analyses of soil and plant tissue
Dried soil and plant tissue samples were analyzed at the Soil Science Laboratory of Sokoine University of Agriculture in Morogoro, Tanzania. Soil texture was determined by the hydrometer method. Soil pH was measured potentiometrically in a 1:2.5 water:soil paste (Klute 1986). Soil organic carbon (SOC) was quantified using the sulfochromic oxidation titration method (Walkley and Black 1934). Total N was extracted from soil and by standard Kjeldahl digest (H2SO4) and quantified via titration (Klute 1986). Available soil nitrate (NO3 −) was determined by potassium chloride (KCl) extraction (Keeney and Nelson 1982). Total P of soil was extracted using concentrated hydrochloric acid (HCl) and nitric acid (HNO3) under reflux; extractants were colorimetrically quantified by the molybdate-blue method using spectrophotometry at 884 nm. Available PO4 −3 was quantified by bicarbonate extraction (Olsen et al. 1954) in soils with a basic pH (SER and SOT). For acidic KUH soil, the extractable Bray-1 P method was used (Bray and Kurtz 1945) and the extractant was quantified using the molybdate-blue method (Murphy and Riley 1962) and a UV VIS spectrophotometer UNICAM 5625 at 884 nm.
Total N in plant shoots was quantified via standard Kjeldahl digest (H2SO4) and titration. Total shoot P was extracted by sulfuric acid (H2SO4) and hydrogen peroxide (H2O2) digest (Klute 1986). To relate P uptake to the amounts of plant-derived C allocated to AM fungal biomass in soil, we calculated the relative mycorrhizal P uptake efficiency as:
where P s is the total amount of P in maize shoots, and C AMF is the amount of C in external AM fungal hyphae (expressed as μg P g C−1).
Data analysis
Experimental responses of plant biomass (total dry weight, root fraction), tissue nutrient content (%N, %P), soil properties (pH, SOC, extractable N and total P) and mycorrhizal measurements (% colonization and total hyphal length density in soil) were analyzed using three-factor ANOVA with soil, water and P as fixed factors. Percent colonization of roots was arcsine square root transformed before analysis. Available soil P was analyzed using two-factor ANOVA for each soil type separately because it was not appropriate to compare data collected using Olsen’s and Bray-1 methods. Means were compared using Fisher’s Protected Least Significant Difference (LSD). Regression analyses were performed to examine relationships between external AM hyphae and soil properties (total P and pH) and test the prediction of the Treseder and Allen model (Fig. 2b). All statistical analyses were conducted using JMP (Version 9. SAS Institute Inc., Cary, NC, USA, 1989–2010) and figures were created using SigmaPlot (Systat Software, San Jose, CA, USA).
Results
Soil characteristics
Soil texture varied considerably across the three soil types (Table 2). Total soil P levels varied as expected across the known P gradient ranging from 0.013 % in KUH soils to 0.33 % in SOT soils (F = 183.00; p < 0.0001); and total soil N was lowest in SER soil (F = 5.21; p = 0.0041; Table 2). At the end of the 5-week experiment, soil from KUH had greater percent soil organic C (SOC;1.62 %) compared to SER and SOT soils (1.28 % and 1.34 %, respectively; F = 13.31; p < 0.0001; Table 3). Soil pH differed across soil types with KUH acidic (6.21), SER neutral (7.44), and SOT basic (8.26; F = 186.28; p < 0.0001). Available NO3 −1 did not vary across soils (mean = 165 mg kg−1; F = 0.21; p = 0.81), Available PO4 −3 differed between SOT (4.51 mg kg−1) and SER (10.17 mg kg−1) soils (F = 49.31; p < 0.0001). Available PO4 −3 of KUH soils was determined to be 5.66 mg kg−1 using a different method, and, therefore, could not be statistically compared to the other soils.
The experimental treatments significantly influenced soil pH and available PO4 −3 (Table 3). Phosphorus fertilizer reduced soil pH by 2.14 % across soil types (F = 4.32; p = 0.040). Ample water decreased available PO4 −3 by 7.19 % across soil types (F = 4.98; p = 0.039), and there was a significant soil x fertilizer interaction. Phosphorus fertilization resulted in a greater than five-fold increase in available PO4 −3 in KUH soil (F = 27.98; p < 0.0001), a 46.6 % increase in SER soil (F = 10.88; p = 0.0028), and no increase in SOT soils (F = 0.11; p = 0.74; Table 4).
Plant responses
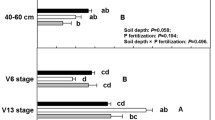
Total dry mass of maize was reduced by 38.9 % in the low water treatment suggesting water-stress was successfully induced (F = 39.10; p < 0.0001; Fig. 3a). Total dry mass of maize did not respond to P fertilization (F = 0.142; p = 0.707) or soil (F = 1.17; p = 0.315); however, root fraction (dry root mass/total dry mass) showed a significant soil x water interaction (F = 9.26; p = 0.0003) and soil x P interaction (F = 3.72; p = 0.0287; Fig. 3b). In the high P treatment, water stress increased plant allocation to roots in SER soil but decreased root fraction in SOT soil. Soil type, water treatment, soil x water and soil x P interactions significantly influenced tissue P (Fig. 3c). Phosphorus fertilization doubled percent tissue P in maize shoots grown in KUH soil from 2.06 % to 4.16 %, but it did not significantly change tissue P in maize grown in the other two soils (F = 9.11; p = 0.0003). In SOT soil, percent tissue P was higher in maize grown in water-limiting treatments (4.82 %) compared to well-watered maize (2.35 %; F = 13.71; p = 0.0004). Soil (F = 11.68; p < 0.0001) and water (F = 4.99; p = 0.029) and their interaction (F = 3.57; p = 0.033) also influenced percent tissue N (Fig. 3d). Tissue N:P ratio was lower in KUH (3.61) than other soils (SER: 5.60; SOT: 6.18; F = 5.15; p = 0.008). Surprisingly, P fertilization did not affect tissue N:P ratio (F = 0.43; p = 0.51), but it was significantly lower in well-watered treatments (F = 5.25; p = 0.025).
Maize biomass, relative allocation to roots, and shoot nutrient content across the experimental treatments: (a) total dry weight, (b) root fraction, (c) tissue P (%), and (d) tissue N (%). Bars represent means ± SE (n = 8). Light bars indicate plants grown under water-limiting conditions and darker represent plants given ample water. Presence of crosshatches represents plants provided supplemental P. Significant factors from three-way ANOVA are indicated by S for soil origin, W for water treatment, and P for P treatment *0.05 ≥ P > 0.01, **0.01 ≥ P > 0.001, ***0.0001 ≥ P. Within a soil type, bars with different letters are significantly different (<0.05) according to Fisher’s Protected LSD test
Mycorrhizal responses
Percent colonization of maize roots by AM fungi was significantly affected by soil type (F = 39.25; p < 0.0001; Fig. 4a); it was lower in SER soil (30.3 %) compared to KUH and SOT soil (62.9 % and 57.1 %, respectively). Across all three soils, mean AM colonization in maize with ample water was 8 % higher than water-stressed maize (F = 10.15; p = 0.002). Similar soil (F = 28.20; p < 0.0001) and water (F = 8.85; p = 0.004) effects were observed for percent root length colonized by arbuscules (data not shown). Unlike root colonization, mean external AM hyphal lengths in SER were nearly double (50.73 m g soil−1) those observed in KUH and SOT (24.75 m g soil−1 and 30.37 m g soil−1, respectively; Fig. 4b; F = 34.39; p < 0.0001). There was a significant soil x P interaction (F = 5.60; p = 0.0054) and nearly a significant soil x water interaction (F = 2.86; p = 0.064). Increased water availability in SER soil significantly increased external hyphal lengths in unfertilized pots. Phosphorus fertilization tended to increase hyphal lengths in KUH and SER soil and significantly decreased external hyphal lengths in SOT soils. The fraction of stained soil hyphae varied between soil types (F = 31.07; p < 0.0001), but was unaffected by other factors (Fig. 5).
Measurements of AM fungi and their relation to plant biomass and nutrient acquisition: (a) total root length colonized by AM fungi, (b) AM hyphal abundance in soil (c) plant C allocation to AM fungi (i.e., the proportion of plant-derived C invested in plant biomass and AM fungal biomass) and (d) mycorrhizal P uptake efficiency (i.e., the amount of P aquired by host plant related to C allocated to AM fungi). Bars represent means ± SE (n = 8). Light bars indicate dry treatments and dark represent amply watered treatments. Presence of crosshatches represents pots provided supplemental P. Significant factors from three-way ANOVA are indicated by S for soil origin, W for water treatment, and P for phosphorus treatment ψ0.1 ≥ P > 0.05, *0.05 ≥ P > 0.01, **0.01 ≥ P > 0.001, and ***0.0001 ≥ P. Within a soil type, bars with different letters are significantly different (<0.05) according to Fisher’s Protected LSD test
Plant C allocation to AM fungi (g fungal C per g plant C) was influenced by a soil x water interaction (F = 6.86; p = 0.0019). Drier treatments in SOT soil increased the biomass of external hyphae per unit plant biomass by four-fold but did not produce a significant change in the other soil types. Relative mycorrhizal P uptake efficiency was greater in KUH soils compared to SOT and SER soils (F = 11.62; p < 0.0001; Fig. 4d).
As predicted by the Treseder and Allen model (Fig. 2b), abundance of external hyphae in soil had a quadratic relationship with total soil P (r2 = 0.81; p < 0.0001; Fig. 6a); external hyphal lengths were positively correlated with total soil P in KUH and SER pots and negatively correlated with total soil P in SOT pots. Similarly, the abundance of external hyphae was positively correlated with Bray-1 available soil PO4 −3 in KUH soils (r2 = 0.22; p = 0.01); and external hyphae had a quadratic relationship with bicarbonate extracted soil PO4 −3 in pooled SER and SOT soils (r2 = 0.34; p < 0.0001). Abundance of AM hyphae in soil also had a quadratic relationship with soil pH (r2 = 0.60; p < 0.0001; Fig. 6b). Acidic and basic soil had lower external hyphal lengths compared to neutral soils.
Quadratic relationships between AM hyphal length and (a) total soil P in pots, and (b) soil pH in pots. Each data point is the mean of a treatment ± SE (n = 8). Squares indicate water-limited treatments, and circles indicate amply watered treatments. Filled shapes represent P-fertilized treatments, and open shapes represent unfertilized treatments
Discussion
Soils in the Serengeti grassland form natural gradients of many physiochemical properties. By growing a uniform host plant (maize) in three different Serengeti soils, we show that soil conditions influence growth of mycorrhizal fungi as well as plant and fungal responses to water availability and P fertilization. We specifically tested three hypotheses about factors that control the formation of mycorrhizas across soil gradients (Table 1). Individually, these hypotheses consider the importance of ambient water, P and soil pH on mycorrhizal formation; however, the hypotheses are not mutually exclusive because interactions among these factors are expected in natural systems. Results of this study support aspects of all three hypotheses. Our findings suggest that mycorrhizas help plants adapt to limiting levels of P and water in the Serengeti. Also, soil pH is related to hyphal densities; this may be a direct effect of pH or an indirect effect related to the influence of soil pH on P availability (Lambers et al. 2012).
If plant investment in AM symbioses was controlled by water availability, then we would predict that water-limiting conditions should increase AM fungal biomass in accordance with the Functional Equilibrium model (H1). Our results only partially support this hypothesis. Mycorrhizal colonization of plant roots and external AM hyphae were not higher in dry conditions; however, the relative amount of C in external hyphae compared to plant C was significantly increased by water stress in SOT soil (Fig. 4c). This suggests enhanced plant investment in AM fungi driven by water stress in soil from the most arid site. It is well established that the influence of mycorrhizas on plant water relations is complicated and arises from both direct and indirect interactions among plants, fungi and soil (Augé 2001, 2004). Miller et al. (1995) observed that drought decreased fine root length and increased AM hyphal length in prairie soils and posited this to be a plant response to a reduced C economy whereby belowground absorptive surface area was increased with less costly AM hyphae. A recent meta-analysis further supports the suggestion that AM symbioses are most beneficial for plant water relations under drought conditions (Augé et al. 2014).
The Treseder and Allen (2002) model (H2) predicts AM fungal abundance as it relates to soil nutrient availability. In very low P soils, AM fungi will be P limited and their abundance is expected to respond positively to P enrichment. Plants grown in soils with ample soil P are expected to invest less C to fungal partners and their AM symbioses are predicted to respond negatively to P enrichment (Fig. 2). Our experimental results support this model with a strong quadratic relationship between total soil P and external hyphae (Fig. 6a). Phosphorus fertilization tended to increase external AM hyphae in pots of KUH and SER soil (Fig. 4b); and, as expected by the Treseder and Allen model, P fertilization reduced hyphal density in SOT soil with high total P levels.
It is interesting that the correlations between external AM hyphae and total soil P are stronger than with extractable soil P. This has been previously observed in a North American grassland (Johnson et al. 1991) and in the Serengeti (Antoninka et al. in preparation), and it suggests that AM fungi access different P pools than those that are traditionally considered to be “available” using standard methods of soil analysis. It is also interesting that P fertilization did not significantly increase available P of SOT soil at the end of the experiment. This suggests that there could be complex interactions with the mineralogy of these soils rich in carboniferous volcanic ash (Oburger et al. 2011).
The absence of positive plant growth response to P fertilization, the magnitude of which was more than adequate to cause a positive growth response in past studies (e.g., Abbott et al. 1984), suggests that the maize plants were not P limited. However, the plants may have been N limited because the mean host plant tissue N:P ratios in our experiment ranged from 3.61 to 6.18, far below 8, the N:P threshold for optimal maize growth in the first growth stage (Jones 1983). Also, N is known to limit grass growth throughout the Serengeti (Seagle and McNaughton 1993), especially the southern region where higher soil P exacerbates N limitation. Because plant N availability affects photosynthetic capacity and consequently the photosynthate available to AM fungi (Johnson 2010), the interactions of soil P and N limitation on AM fungal dynamics need to be further disentangled experimentally.
As predicted by H3, AM hyphal densities varied with soil pH, they were higher at SER, where soil pH is close to neutral compared to KUH and SOT with acidic and basic soils, respectively (Table 3; Fig. 6b). Other studies have shown that AM fungal abundance increases with pH up to 7.5 (Abbott and Robson 1985; Johnson et al. 1991; Coughlan et al. 2000). Few studies of pH responses of AM abundance examine such a broad pH range as this study. Our findings suggest acidity and alkalinity thresholds where AM hyphal production is reduced possibly because P and other nutrients are more strongly occluded in acidic and alkaline soils (Brady and Weil 1996). The soil pH gradient is superimposed on the P fertility gradient across the Serengeti, and both factors may affect plant and AM fungal nutritional status. Experimentally uncoupling these factors would reveal their individual effects on AM fungal dynamics.
There was twice as much AM hyphae recovered from pots of SER soil compared to other soils, yet percent AM root colonization was the lowest in this soil type. Taxa of AM fungi differ in the proportion of their biomass that forms inside and outside roots (Hart and Reader 2002; Thonar et al. 2011). Ongoing studies indicate that the community composition of AM fungi varies across the Serengeti’s precipitation/fertility gradient (Antoninka et al. in preparation). Consequently, different species of AM fungi may be responsible for differences in the extent of root colonization and external AM hyphae present in pots. Proportion of hyphae observed that were stained (Fig. 5) could be indicative of different AM fungal community composition because the affinity for trypan blue stain has been demonstrated to vary among AM fungal taxa (Dodd et al. 2000).
Our study of AM fungal communities from three sites with vastly different soil properties in the Serengeti supports the hypothesis that AM symbiosis help plants adapt to their local environmental conditions. Different AM fungal taxa are known to be viable and infective in a different pH ranges (Porter et al. 1987; Brundrett 1991; van Aarle et al. 2002) possibly contributing to allopatric divergence and local adaptation of AM fungi to specific soil properties and host plants throughout the Serengeti. In other systems, AM fungal ecotypes from P limited locales provide greater P-uptake benefit compared to ecotypes from P rich soil (Johnson et al. 2010). Similarly, fungal isolates from dry sites better alleviate host water stress compared to isolates from mesic sites (Stahl et al. 1984; Bethlenfalvay et al. 1989). The mycorrhizal uptake efficiency of P was much greater for host plants grown in P limited KUH soil (Fig. 4d). This may indicate that the amount of plant-derived C invested belowground is more tightly linked to the acquisition of soil P in KUH soil. In SOT soil where precipitation is lowest, when plant biomass is standardized, as in Fig. 4c, there is more fungal biomass per unit plant in response to plant water limitation. This has potential importance because it suggests that the fungi in this drier site can form relatively more biomass under dry conditions compared to wet conditions.
Mycorrhizal symbioses vary across precipitation and soil gradients in the Serengeti. Our water-stress and P fertilization experiment supports the hypothesis that AM associations may help plants overcome limitation of belowground resources. We show that plant nutritional status, determined by ambient soil fertility and water availability, influences the formation of AM fungi inside and outside plant roots. Both the Functional Equilibrium model and the Treseder & Allen model are useful for predicting the dynamics of AM fungal biomass across resource gradients. Precipitation, P, pH, and soil types interact to yield a complex mosaic of edaphic conditions across the Serengeti ecosystem. Such diversity may facilitate local AM fungal adaptation to specific soil properties and selection of ecotypes that most efficiently forage for the most limiting resource.
References
Abbott LK, Robson AD (1985) The effect of soil pH on the formation of VA mycorrhizas by two species of Glomus. Soil Res 23:253–261
Abbott LK, Robson AD, de Boer G (1984) The effect of phosphorus on the formation of hyphae in soil by the vesicular-arbuscular mycorrhizal fungus, Glomus fasciculatum. New Phytol 97:437–446
Al Karaki GN, Clark RB (1998) Growth, mineral acquisition, and water use by mycorrhizal wheat grown under water stress. J Plant Nutr 21:263–276
Amos B, Walters DT (2006) Maize root biomass and net rhizodeposited carbon. Soil Sci Soc Am J 70:1489–1503
Anderson GD, Talbot LM (1965) Soil factors affecting the distribution of the grassland types and their utilization by wild animals on the Serengeti Plains, Tanganyika. J Ecol 52:33–56
Anderson RC, Liberta AE, Dickman LA (1984) Interaction of vascular plants and vesicular-arbuscular mycorrhizal fungi across a soil moisture-nutrient gradient. Oecologia 64:111–117
Anderson TM, Ritchie ME, McNaughton SJ (2007) Rainfall and soils modify plant community response to grazing in Serengeti National Park. Ecol 88:1191–1201
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42
Augé RM (2004) Arbuscular mycorrhizae and soil/plant water relations. Can J Soil Sci 84:373–381
Augé RM, Toler HD, Saxton AM (2014) Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: a meta-analysis, Mycorrhiza 1–12 DOI 10.1007/s00572-014-0585-4
Bethlenfalvay GJ, Bayne HG, Pacovsky RS (1983) Parasitic and mutualistic associations between a mycorrhizal fungus and soybean: the effect of phosphorus on host plant endophyte interactions. Physiol Plant 57:543–548
Bethlenfalvay GJ, Franson RL, Brown MS, Mihara KL (1989) The glycine Glomus Bradyrhizobium symbiosis. IX. Nutritional, morphological and physiological responses of nodulated soybean to geographic isolates of the mycorrhizal fungus Glomus mosseae. Physiol Plant 76:226–232
Blanke V, Wagner M, Renker C, Lippert H, Michulitz M, Kuhn AJ, Buscot F (2011) Arbuscular mycorrhizas in phosphate-polluted soil: interrelations between root colonization and nitrogen. Plant Soil 343:379–392
Bolan NS (1991) A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants. Plant Soil 134:189–207
Brady NC, Weil RR (1996) Elements of the nature and properties of soils. Prentice Hall, Upper Saddle River
Bray RH, Kurtz LT (1945) Determination of total, organic, and available forms of phosphorus in soils. Soil Sci 59:39–46
Brouwer R (1983) Functional equilibrium: sense or nonsense?[root/shoot ratio]. Neth J Agric Sci 31:335–348
Brundrett M (1991) Mycorrhizas in natural ecosystems. Adv Ecol Res 21:171–313
Coughlan AP, Dalpé Y, Lapointe L, Piché Y (2000) Soil pH-induced changes in root colonization, diversity, and reproduction of symbiotic arbuscular mycorrhizal fungi from healthy and declining maple forests. Can J For Res 30:1543–1554
Cuenca G, De Andrade Z, Lovera M, Fajardo L, Meneses E (2003) Mycorrhizal response of Clusia pusilla growing in two different soils in the field. Trees 17:200–206
Dodd JC, Boddington CL, Rodriguez A, Gonzalez-Chavez C, Mansur I (2000) Mycelium of arbuscular mycorrhizal fungi (AMF) from different genera: form, function and detection. Plant Soil 226:131–151
Fellbaum CR, Mensah JA, Pfeffer PE, Kiers ET, Bücking H (2012) The role of carbon in fungal nutrient uptake and transport: implications for resource exchange in the arbuscular mycorrhizal symbiosis. Plant Signal Behav 7
Fitter AH, Heinemeyer A, Olsen E (2004) System responses to environmental change: the mycorrhizal component. Can J Bot 82:1–7
Frank DA, McNaughton SJ, Tracy BF (1998) The ecology of the earth's grazing ecosystems. BioSci 48:513–521
Friese CF, Allen MF (1991) The spread of VA mycorrhizal fungal hyphae in the soil: inoculum types and external hyphal architecture. Mycologia 83:409–418
George E, Marschner H, Jakobsen I (1995) Role of arbuscular mycorrhizal fungi in uptake of phosphorus and nitrogen from soil. Crit Rev Biotechnol 15:257–270
Hammer EC, Pallon J, Wallander H, Olsson PA (2011) Tit for tat? A mycorrhizal fungus accumulates phosphorus under low plant carbon availability. FEMS Microbiol Ecol 76:236–244
Hart MM, Reader RJ (2002) Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol 153:335–344
Hernandez RR, Allen MF (2013) Diurnal patterns of productivity of arbuscular mycorrhizal fungi revealed with the soil ecosystem observatory. New Phytol 200:547–557
Jakobsen I, Abbott LK, Robson AD (1992) External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. 1. Spread of hyphae and phosphorus inflow into roots. New Phytol 20:371–379
Johnson NC, Zak DR, Tilman D, Pfleger FL (1991) Dynamics of vesicular-arbuscular mycorrhizae during old field succession. Oecologia 86:349–358
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytol 135:575–585
Johnson NC, Rowland DL, Corkidi L, Egerton-Warburton LM, Allen EB (2003) Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecol 84:1895–1908
Johnson NC, Wilson GW, Bowker MA, Wilson JA, Miller RM (2010) Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc Natl Acad Sci U S A 107:2093–2098
Johnson NC, Antoninka, A, Ritchie, M (2013) Glomeromycota of the Serengeti: abundance and community composition are influenced by rainfall and soil properties [abstract]. In: 7th International Conference on Mycorrhiza. 6–11 January 2013 New Dehli, India p 38
Jones CA (1983) A survey of the variability in tissue nitrogen and phosphorus concentrations in maize and grain sorghum. Field Crops Res 6:133–147
Keeney DR, Nelson DW (1982) Nitrogen: Inorganic forms. In: Page AL et al. (eds.) Methods of soil analysis. Part 2. Chemical and microbiological properties, 2nd ed. SSSA Book Ser. 5. SSSA, Madison, USA, pp 643–698
Klironomos JN (2003) Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecol 84:2292–2301
Klute A (1986) Methods of soil analysis part I. In: Klute A (ed) Physical and mineralogical methods. American Society of Agronomy, Madison, pp 635–662
Koide RT (1991) Tansley Review No. 29. Nutrient supply, nutrient demand and plant response to mycorrhizal infection. New Phytol 117:365–386
Lambers H, Bishop JG, Hopper SD, Laliberté E, Zúñiga-Feest A (2012) Phosphorus-mobilization ecosystem engineering: the roles of cluster roots and carboxylate exudation in young P-limited ecosystems. Ann Bot 110:329–348
Latshaw WL, Miller EC (1924) Elemental composition of the corn plant. J Agric Res 27:845–861
Liu Y, Shi G, Mao L, Cheng G, Jiang S, Ma X, An L, Du G, Johnson NC, Feng H (2012) Direct and indirect influences of 8 yr of nitrogen and phosphorus fertilization on Glomeromycota in an alpine meadow ecosystem. New Phytol 194:523–535
Marschner H, Dell B (1994) Nutrient uptake in mycorrhizal symbiosis. Plant Soil 159:89–102
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501
McNaughton SJ (1983) Serengeti grassland ecology: the role of composite environmental factors and contingency in community organization. Ecol Monogr 53:291–320
McNaughton SJ (1985) Ecology of a grazing ecosystem: the Serengeti. Ecol Monogr 55:259–294
McNaughton SJ (1990) Mineral nutrition and seasonal movements of African migratory ungulates. Nature 345:613–615
Miller RM, Jastrow JD, Reinhardt DR (1995) External hyphal production of vesicular-arbuscular mycorrhizal fungi in pasture and tallgrass prairie communities. Oecologia 103:17–23
Miller RM, Wilson GW, Johnson NC (2012) Arbuscular mycorrhizae and grassland ecosystems. In: Southworth D (ed) Biocomplexity of plant-fungal interactions. Wiley, Hoboken, pp 59–84
Mosse B, Hayman DS, Arnold DJ (1973) Plant growth responses to vesicular-arbuscular mycorrhiza. V. Phosphate uptake by three plant species from P-deficient soils labeled with 32P. New Phytol 72:809–815
Msolla MM, Semoka JMR, Borggaard OK (2005) Hard minjingu phosphate rock: an alternative P source for maize production on acid soils in Tanzania. Nutr Cycl Agroecosyst 72:299–308
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Oburger E, Jones DL, Wenzel WW (2011) Phosphorus saturation and pH differentially regulate the efficiency of organic acid anion-mediated P solubilization mechanisms in soil. Plant Soil 341:363–382
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circular 939, US Government Printing Office, Washington DC
Olsson PA, Johnson NC (2005) Tracking carbon from the atmosphere to the rhizosphere. Ecol Lett 8:1264–1270
Peng S, Eissenstat DM, Graham JH, Williams K, Hodge NC (1993) Growth depression in mycorrhizal citrus at high-phosphorus supply (analysis of carbon costs). Plant Physiol 101:1063–1071
Porter WM, Robson AD, Abbott LK (1987) Field survey of the distribution of vesicular-arbuscular mycorrhizal fungi in relation to soil pH. J Appl Ecol 24:659–662
Rillig MC, Field CB, Allen MF (1999) Soil biota responses to long-term atmospheric CO2 enrichment in two California annual grasslands. Oecologia 119:572–577
Ruess RW, McNaughton SJ (1987) Grazing and the dynamics of nutrient and energy regulated microbial processes in the Serengeti grasslands. Oikos 49:101–110
Ruess RW, Seagle SW (1994) Landscape patterns in soil microbial processes in the Serengeti National Park, Tanzania. Ecol 75:892–904
Sanginga N, Okogun JA, Akobundu IO, Kang BT (1996) Phosphorus requirement and nodulation of herbaceous and shrub legumes in low P soils of a Guinean savanna in Nigeria. Appl Soil Ecol 3:247–255
Seagle SW, McNaughton SJ (1993) Simulated effects of precipitation and nitrogen on Serengeti grassland productivity. Biogeochem 22:157–178
Staddon PL, Ramsey CB, Ostle N, Ineson P, Fitter AH (2003) Rapid turnover of hyphae of mycorrhizal fungi determined by AMS microanalysis of 14C. Sci 300:1138–1140
Stahl PD, Smith WK (1984) Effects of different geographic isolates of Glomus on the water relations of Agropyron smithii. Mycologia 76:261–267
Steinberg PD, Rillig MC (2003) Differential decomposition of arbuscular mycorrhizal fungal hyphae and glomalin. Soil Biol Biochem 35:191–194
Thonar C, Schnepf A, Frossard E, Roose T, Jansa J (2011) Traits related to differences in function among three arbuscular mycorrhizal fungi. Plant Soil 339:231–245
Treseder KK, Allen MF (2002) Direct nitrogen and phosphorus limitation of arbuscular mycorrhizal fungi: a model and field test. New Phytol 155:507–515
Treseder KK, Schimel JP, Garcia MO, Whiteside MD (2010) Slow turnover and production of fungal hyphae during a Californian dry season. Soil Biol Biochem 42:1657–1660
Van Aarle IM, Olsson PA, Söderström B (2002) Arbuscular mycorrhizal fungi respond to the substrate pH of their extraradical mycelium by altered growth and root colonization. New Phytol 155:173–182
Vierheilig H, Coughlan AP, Wyss U, Piché Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 64:5004–5007
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecol Appl 20:5–15
Walker TW, Syers JK (1976) The fate of phosphorus during pedogenesis. Geoderma 15:1–19
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 34:29–38
Wu QS, Xia RX (2006) Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J Plant Physiol 163:417–425
Acknowledgments
This work was supported by the National Science Foundation (DEB-0842327). We thank Tanzanian National Parks (TANAPA) and the Tanzanian Wildlife Institute Research Institute (TAWIRI) for permitting us to conduct this research, and Sokoine University of Agriculture in Morogoro for hosting us and analyzing soil and plant samples. We also thank Emilian Mayemba and Geofrey Soka for help in the field, Kelly Fruth for extracting hyphae from soil and making slides, Julie Wolfe and Michael Miller for developing hyphal length and biomass calculations, Anita Antoninka and Daniel Revillini for their statistical guidance, and Bo Stevens for supplying us with Fig. 1. Additionally, we are thankful to two anonymous reviewers for their insight and advice to improve this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Peter Christie.
Rights and permissions
About this article
Cite this article
Propster, J.R., Johnson, N.C. Uncoupling the effects of phosphorus and precipitation on arbuscular mycorrhizas in the Serengeti. Plant Soil 388, 21–34 (2015). https://doi.org/10.1007/s11104-014-2369-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2369-1