Abstract
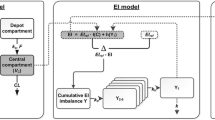
Energy intake (EI) is a pivotal biomarker used in quantification approaches to metabolic disease processes such as obesity, diabetes, and growth disorders. Eating behavior is however under both short-term and long-term control. This control system manifests itself as tolerance and rebound phenomena in EI, when challenged by drug treatment or diet restriction. The paper describes a model with the capability to capture physiological counter-regulatory feedback actions triggered by energy imbalances. This feedback is general as it handles tolerance to both increases and decreases in EI, and works in both acute and chronic settings. A drug mechanism function inhibits (or stimulates) EI. The deviation of EI relative to a reference level (set-point) serves as input to a non-linear appetite control signal which in turn impacts EI in parallel to the drug intervention. Three examples demonstrate the potential usefulness of the model in both acute and chronic dosing situations. The model shifts the predicted concentration–response relationship rightwardly at lower concentrations, in contrast to models that do not handle functional adaptation. A fourth example further shows that the model may qualitatively explain differences in rate and extent of adaptation in observed EI and its concomitants in both rodents and humans.









Similar content being viewed by others
References
Goodman LS, Gilman A (1996) The pharmacological basis of therapeutics, 11th edn. McGrawHill, New York
Sällström B, Visser SA, Forsberg T, Peletier LA, Ericson AC, Gabrielsson J (2005) A pharmacodynamic turnover model capturing asymmetric circadian baselines of body temperature, heart rate and blood pressure in rats: challenges in terms of tolerance and animal handling effects. J Pharmacokinet Pharmacodyn 32(5–6):835–859
Ahlström C, Peletier LA, Gabrielsson J (2011) Quantitative analysis of rate and extent of tolerance of biomarkers: application to nicotinic acid-induced changes in non-esterified fatty acids in rats. Eur J Pharm Sci 44(3):250–264
Ahlström C, Peletier LA, Jansson-Löfmark R, Gabrielsson J (2011) Feedback modeling of non-esterified fatty acids in rats after nicotinic acid infusions. J Pharmacokinet Pharmacodyn 38(1):1–24
Ahlström C, Kroon T, Peletier LA, Gabrielsson J (2013) Feedback modeling of non-esterified fatty acids in obese Zucker rats after nicotinic acid infusions. J Pharmacokinet Pharmacodyn 40(6):623–638
Ahlström C, Peletier LA, Gabrielsson J (2013) Challenges of a mechanistic feedback model describing nicotinic acid-induced changes in non-esterified fatty acids in rats. J Pharmacokinet Pharmacodyn 40(4):497–512
Torgerson JS, Hauptman J, Boldrin MN, Sjöström L (2004) XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 27(1):155–161
Kang JG, Park CY (2012) Anti-obesity drugs: a review about their effects and safety. Diabetes Metab J 36(1):13–25
Jusko WJ, Ko HC, Ebling WF (1995) Convergence of direct and indirect pharmacodynamic response models. J Pharmacokinet Biopharm 23:5–8
Danhof M, Alvan G, Dahl SG, Kuhlmann J, Paintaud G (2005) Mechanism-based pharmacokinetic-pharmacodynamic modeling—a new classification of biomarkers. Pharm Res 22(9):1432–1437
Woods SC, D’Alessio DA (2008) Central control of body weight and appetite. J Clin Endocrinol Metab 93(11 Suppl 1):S37–S50
Lenard NR, Berthoud HR (2008) Central and peripheral regulation of food intake and physical activity: pathways and genes. Obesity (Silver Spring) 16(Suppl 3):S11–S22
Fernstrom JD, Choi S (2008) The development of tolerance to drugs that suppress food intake. Pharmacol Ther 117(1):105–122
Tam J, Fukumura D, Jain RK (2009) A mathematical model of murine metabolic regulation by leptin: energy balance and defense of a stable body weight. Cell Metab 9(1):52–63
Speakman JR, Levitsky DA, Allison DB, Bray MS, de Castro JM, Clegg DJ, Clapham JC, Dulloo AG, Gruer L, Haw S, Hebebrand J, Hetherington MM, Higgs S, Jebb SA, Loos RJ, Luckman S, Luke A, Mohammed-Ali V, O’Rahilly S, Pereira M, Perusse L, Robinson TN, Rolls B, Symonds ME, Westerterp-Plantenga MS (2011) Set points, settling points and some alternative models: theoretical options to understand how genes and environments combine to regulate body adiposity. Dis Model Mech 4(6):733–745
de Graaf C, Blom WA, Smeets PA, Stafleu A, Hendriks HF (2004) Biomarkers of satiation and satiety. Am J Clin Nutr 79(6):946–961
Reidelberger R, Haver A, Chelikani PK, Apenteng B, Perriotte-Olson C, Anders K, Steenson S, Blevins JE (2012) Effects of leptin replacement alone and with exendin-4 on food intake and weight regain in weight-reduced diet-induced obese rats. Am J Physiol Endocrinol Metab 302(12):E1576–E1585
Keys A, Brozek J, Henschel A, Mickelsen O, Taylor HL (1950) The biology of human starvation. University of Minnesota Press, Minneapolis
Dulloo AG, Jacquet J, Girardier L (1997) Poststarvation hyperphagia and body fat overshooting in humans: a role for feedback signals from lean and fat tissues. Am J Clin Nutr 65(3):717–723
Inoue K, Kiriike N, Fujisaki Y, Kurioka M, Yamagami S (1997) Effects of fluvoxamine on food intake during rebound hyperphagia in rats. Physiol Behav 61(4):603–608
Lauzurica N, Garca-Garca L, Pinto S, Fuentes JA, Delgado M (2010) Changes in NPY and POMC, but not serotonin transporter, following a restricted feeding/repletion protocol in rats. Brain Res 1313:103–112
Kievit P, Halem H, Marks DL, Dong JZ, Glavas MM, Sinnayah P, Pranger L, Cowley MA, Grove KL, Culler MD (2012) Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes 62(2):490–497
Gennemark P, Jansson-Löfmark R, Hyberg G, Wigstrand M, Kakol-Palm D, Håkansson P, Hovdal D, Brodin P, Fritsch-Fredin M, Antonsson M, Ploj K, Gabrielsson J (2013) A modeling approach for compounds affecting body composition. J Pharmacokinet Pharmacodyn 40(6):651–667
Kennedy GC (1953) The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B Biol Sci 140(901):578–596
Glad T, Ljung L (2000) Control theory: multivariable and nonlinear methods. Taylor & Francis, London
Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR (2001) Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 86(12):5992
Fang J, DuBois DC, He Y, Almon RR, Jusko WJ (2011) Dynamic modeling of methylprednisolone effects on body weight and glucose regulation in rats. J Pharmacokinet Pharmacodyn 38(3):293–316
Yuan J (1993) Modeling blood/plasma concentrations in dosed feed and dosed drinking water toxicology studies. Toxicol Appl Pharmacol 119(1):131–141
Ellacott KL, Morton GJ, Woods SC, Tso P, Schwartz MW (2010) Assessment of feeding behavior in laboratory mice. Cell Metab 12(1):10–17
Gabrielsson J, Weiner D (2010) Pharmacokinetic & pharmacodynamic, data analysis, concepts and applications, 4th edn, 2nd print. Swedish Pharmaceutical Press, Stockholm. ISBN-139789197651004
Laird AK (1964) Dynamics of tumor growth. Br J Cancer 18(3):490–502
Le Magnen J, Tallon S (1966) La priodicit spontane de la prise d’aliments ad libitum du rat blanc. J Physiol (Paris) 58:323–349
Del Prete E, Scharrer E (1993) Influence of age and hepatic branch vagotomy on the night/day distribution of food intake in rats. Z Ernahrungswiss 32(4):316–320
Reinsch C (1967) Smoothing by spline functions. Numer Math 10:177–183
Fisas A, Codony X, Romero G, Dordal A, Giraldo J, Merc R, Holenz J, Vrang N, Srensen RV, Heal D, Buschmann H, Pauwels PJ (2006) Chronic 5-HT6 receptor modulation by E-6837 induces hypophagia and sustained weight loss in diet-induced obese rats. Br J Pharmacol 148(7):973–983
Mulla MSA, Goyal VK, Jana S, Nirogi R (2010) Safety evaluation of sibutramine in Wistar rats. Afr J Basic Appl Sci 2(5–6):128–134
Li DX, Jang KY, Kang W, Bae K, Lee MH, Oh YK, Jee JP, Park YJ, Oh DH, Seo YG, Kim YR, Kim JO, Woo JS, Yong CS, Choi HG (2010) Enhanced solubility and bioavailability of sibutramine base by solid dispersion system with aqueous medium. Biol Pharm Bull 33(2):279–284
Hansen DL, Toubro S, Stock MJ, Macdonald IA, Astrup A (1999) The effect of sibutramine on energy expenditure and appetite during chronic treatment without dietary restriction. Int J Obes Relat Metab Disord 23(10):1016–1024
Vickers SP, Jackson HC, Cheetham SC (2011) The utility of animal models to evaluate novel anti-obesity agents. Br J Pharmacol 164(4):1248–1262
Colburn WA, Eldon MA (1994) Simultaneous pharmacokinetic/pharmacodynamic modeling. In: Butler NR, Sramek JJ, Narang PK (eds) Pharmacodynamics and drug development: perspectives in clinical pharmacology. Wiley, New York
Porchet HC, Benowitz NL, Sheiner LB (1988) Pharmacodynamic model of tolerance: application to nicotine. J Pharmacol Exp Ther 244:231–236
Ouellet DMC, Pollack GM (1997) Pharmacodynamics and tolerance development during multiple administrations in rats. J Pharmacol Exp Ther 281:713–720
Urquhart J, Li CC (1968) The dynamics of adrenocortical secretion. Am J Physiol 214:73–85
Urquhart J, Li CC (1969) Dynamic testing and modeling of andrecortical secretory function. Ann N Y Acad Sci 156:756–778
Veng-Pederson P, Modi NB (1993) A system approach to pharmacodynamics. Input-effect control system analysis of central nervous system effect of alfentanil. J Pharm Sci 82:266–272
Licko V, Ekblad EB (1992) Dynamics of a metabolic system: what single-action agents reveal about acid secretion. Am J Physiol 262:G581–G592
Bauer JA, Fung HL (1994) Pharmacodynamic models of nitroglycerin-induced hemodynamic tolerance in experimental heart failure. Pharm Res 11:816–823
Sharma A, Ebling WF, Jusko WJ (1998) Precursor-dependent indirect pharmacodynamic response model for tolerance and rebound phenomena. J Pharm Sci 87:1577–1584
Ackerman E, Rosevear JW, McGuckin WF (1964) A mathematical model of the glucose-tolerance test. Phys Med Biol 9:203–213
Rescigno A, Segre G (1966) Drug and tracer kinetics. Blaisdell Publishing Company, London
Wakelkamp M, Alvan G, Gabrielsson J, Paintaud G (1996) Pharmacodynamic modeling of furosemide tolerance after multiple intravenous administration. Clin Pharmacol Ther 60:75–88
Agersø H, Ynddal L, Søgaard B, Zdravkovic M (2001) Pharmacokinetic and pharmacodynamic modeling of NN703, a growth hormone Secretagogue, after a single po dose to human volunteers. J Clin Pharmacol 41:163–169
Zuideveld KP, Maas HJ, Treijtel N, Hulshof J, van der Graaf PH, Peletier LA, Danhof M (2001) A set-point model with oscillatory behavior predicts the time course of 8-OH-DPAT-induced hypothermia. Am J Physiol Regul Integr Comp Physiol 281:R2059–R2071
Lima JJ, Matsushima N, Kissoon N, Wang J, Sylvester JE, Jusko WJ (2004) Modeling the metabolic effects of terbutaline in β2-adrenergic receptor diplotypes. Clin Pharmacol Ther 76:27–37
de Winter W, DeJongh J, Post T, Ploeger B, Urquhart R, Moules I, Eckland D, Danhof M (2006) A mechanism-based disease progression model for comparison of long-term effects of pioglitazone, metformin and gliclazide on disease processes underlying type 2 diabetes mellitus. J Pharmacokinet Pharmacodyn 33(3):313–343
Goldbeter A (1995) A model for circadian oscillations in the Drosophila period protein (PER). Proc Biol Sci 261(1362):319–324
Yi TM, Huang Y, Simon MI, Doyle J (2000) Robust perfect adaptation in bacterial chemotaxis through integral feedback control. Proc Natl Acad Sci U S A 97(9):4649–4653
Gennemark P, Nordlander B, Hohmann S, Wedelin D (2006) A simple mathematical model of adaptation to high osmolarity in yeast. In Silico Biol 6:0018
Muzzey D, Gómez-Uribe CA, Mettetal JT, van Oudenaarden A (2009) A systems-level analysis of perfect adaptation in yeast osmoregulation. Cell 138(1):160–171
He F, Fromion V, Westerhoff HV (2013) (Im)Perfect robustness and adaptation of metabolic networks subject to metabolic and gene-expression regulation: marrying control engineering with metabolic control analysis. BMC Syst Biol 7:131
Thomsen WJ, Grottick AJ, Menzhagi F, Reyes-Saldana H, Espitia S, Yuskin D, Whelan K, Martin M, Morgan M, Chen W, Al-Shamma H, Smith B, Behan D (2008) Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther 325:577–587
Vickers SP, Easton N, Webster LJ, Wyatt A, Bickerdike MJ, Dourish CT, Kennett GA (2003) Oral administration of the \(\text{5-HT }_{2C}\) receptor agonist, \(m\)CPP, reduces body weight gain in rats over 28 days as a result of maintained hypophagia. Psychopharmacology (Berl) 167(3):274–280
Hall KD (2011) Quantification of the effect of energy imbalance on bodyweight. Lancet 378:826–837
Grimwood S, Hartig PR (2009) Target site occupancy: emerging generalizations from clinical and preclinical studies. Pharmacol Ther 122:281–301
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
Basic model, step 2
Several alternatives to Eq. 1 can be considered. In case a basal level \(p_0\) is anticipated one can alternatively use
Another possibility is to delay the peak EI from \(t=0\) to \(t=1/p_2\) by the function
or by the Bateman function
Extended model, step 6
We model the appetite control signal \(h(Y)\) as a function of the cumulative energy imbalance function \(Y\) by the Gompertz function \(h(Y)\) defined as
where the parameter \(h_{disp}\) is a negative number that sets the displacement on the y-axis, and the parameter \(h_{slope}\) sets the rate to steady-state, both during treatment and of the overshoot.
When the cumulative energy imbalance function is zero, \(Y(t)=0\), there is no modulation of EI, \(h(Y)=1\). The constraint \(Y=0 \rightarrow h(Y)=1\) gives
and \(h(Y)\) is defined with the three parameters \(h_{\rm min},\,h_{\rm max}\), and \(h_{slope}\) as
Steady-state calculation
For the perfect adaptation case, when \(k=0\), the utility during treatment is defined as follows. First, from Eq. 10 we see that the relative change in EI rate is defined by the product
that expands to
At steady-state EI during treatment, \(Y\), representing the cumulative appetite goes to a large number, and Eq. 20 reduces to
If this value is equal to (or greater than) one, the drug provocation is matched by the feedback and the system reaches a steady-state where EI is the same as for the reference vehicle. If the value is less than one, the drug provocation is stronger than the maximum feedback, and steady-state EI is less than that of the vehicle. For example, if \(I(C)=0.25\) and \(h_{\rm max}=3\), then EI is initially reduced to 25 % (as \(I(C)=0.25\)) and then gradually reaches 75 % (the product of \(I(C)\) and \(h(Y)\) equals 0.75) at steady-state during treatment.
The utility of a drug treatment, defined as treatment effect area divided by overshoot area, mainly depends on the parameter \(k\). A large \(k\) value results in high utility. The special case \(k=0\) gives perfect adaptation with no utility of the drug treatment (utility = 1). Utility is also influenced by e.g. \(h_{slope}\) and treatment length.
At steady-state, Eq. 8 gives
Considering average \(EI_{veh}\) and steady-state \(C\), we can numerically approximate \(h(Y)\) versus steady-state concentration from the model.
Parameter sensitivity
Sensitivity of the error function (sum-of-squared residuals) to variations in the regressed parameters of Example 2–4 are reported in Table 6. In particular, the parameter \(h_{slope}\) is insensitive using available data.
Rights and permissions
About this article
Cite this article
Gennemark, P., Hjorth, S. & Gabrielsson, J. Modeling energy intake by adding homeostatic feedback and drug intervention. J Pharmacokinet Pharmacodyn 42, 79–96 (2015). https://doi.org/10.1007/s10928-014-9399-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-014-9399-4