Abstract
The diverse and densely populated gastrointestinal microbiota is essential for the regulation of host physiology and immune function. As our knowledge of the composition and function of the intestinal microbiota continues to expand, there is new interest in using these developments to tailor fecal microbiota transplantation (FMT) and microbial ecosystem therapeutics (MET) for a variety of diseases. The potential role of FMT and MET in the treatment of Clostridium difficile infection (CDI)—currently the leading nosocomial gastrointestinal infection—has proven highly effective for recurrent CDI, and has emerged as a paradigm shift in the treatment of this disease. The current review will serve as a summary of the key aspects of CDI, and will introduce the essential framework and challenges of FMT, as is currently practiced. MET represents the progression of conventional bacteriotherapy that fundamentally capitalizes on the restorative properties of intestinal bacterial communities and may be viewed as the culmination of a rationally designed therapeutic modality. As our understanding of the composition and function of the intestinal microbiota evolves, it will likely drive next-generation microbiota therapies for a range of medical conditions, such as inflammatory bowel disease, obesity, and metabolic syndrome.
Similar content being viewed by others
Introduction
The gastrointestinal tract houses one of the most diverse and densely populated microbial ecosystems on the planet [1]. The complex gastrointestinal microbiota plays a pivotal role in the regulation of host physiology, and certain bacteria including Bacteroides, Bifidobacterium, Clostridium clusters XIVa and IVa, Eubacterium, Faecalibacterium, Lactobacillus, and Roseburia have been associated with gastrointestinal health [2]. As technological advances in bioinformatics, metagenomic analyses, and germ-free models have expanded our knowledge of the intestinal microbiota composition and function, this has led to the application of fecal microbiota transplantation (FMT) and intestinal microbiota therapeutics for a variety of diseases. Clostridium difficile infection (CDI) and the use of FMT and microbial ecosystem therapeutics (MET) as a successful treatment for recurrent CDI will be the focus of this review.
Clostridium difficile infection (CDI)
Clostridium difficile infection (CDI) is a gastrointestinal disease caused by C. difficile, a Gram-positive, spore-forming and toxin-producing anaerobic bacillus. CDI is a highly prevalent nosocomial infection and a leading cause of diarrhea and abdominal pain, accounting for 15–25 % of antibiotic-associated diarrhea. If left untreated, CDI can progress to pseudomembranous colitis, toxic megacolon, and death [3]. C. difficile was originally identified in 1935 as an innocuous normal gut microbe from the infant gastrointestinal tract, and has since evolved to become an opportunistic pathogen of epidemic proportions among individuals whose normal gut microbiota has been functionally altered [4–6].
In the United States, recent reports have shown an incidence of CDI of 84 per 100,000 persons, with associated acute hospital care expenditures estimated in the range of USD 1.2–5.9 billion [7, 8]. Moreover, C. difficile is an emerging global health burden, with rising incidence in Asian countries, where it was previously found in lower proportions [9]. The dramatic increase in the prevalence of CDI over the last decade can be attributed in part to the emergence of the hypervirulent NAP1/ribotype027 epidemic strains in North America, which subsequently spread to Europe, Australia, and Japan [10, 11]. Also of growing concern is the increasing incidence of CDI in children as well as in pregnant women [12, 13], and an increase in community-associated CDI, including among young adults [14]. For instance, rates of community-acquired CDI among the general population have been found to vary between 3.2 and 50 cases per 100,000 [14, 15]. Approximately 40 % of patients acquiring community-associated CDI are reported to have no prior antibiotic exposure; Chitnis et al. reported a rate of 36 % in their multi-center interview data [14, 16]. In addition to the majority of patients with community-associated CDI having had antibiotic exposure within 12 weeks, the authors found that 82 % of this cohort had outpatient or inpatient health care exposure, and there was a trend toward proton pump inhibitor (PPI) use, compared to those without antibiotic exposure [14]. Contact with children younger than 2 years, who are known to be frequent asymptomatic carriers of C. difficile, has also been associated with increased risk of community-acquired CDI [14, 17, 18]. The pathophysiology underlying the association of CDI with these lesser-known yet emerging cohorts remains unclear. Other risk factors that have been linked with CDI are age greater than 65 years, proton pump inhibitor use, prolonged hospital stay, and severe comorbidities such as inflammatory bowel disease and chronic renal disease [19–27].
While the pathogenesis of C. difficile is not entirely clear, it is well recognized that functional alterations in the gastrointestinal microbiota create a conducive environment for C. difficile sporulation, spore germination, and production of toxins that contribute to CDI. The source reservoir of C. difficile, either zoonotic or common environmental source, has yet to be confirmed [28]. However, there is growing concern about the role of animals as a reservoir, given that certain ribotypes, such as ribotype 078 are commonly found in animals [29].
Recurrent C. difficile infection (rCDI)
Recurrent CDI (rCDI) refers to the clinical resolution of CDI while on appropriate therapy, followed by relapse of infection after treatment has been completed [30]. While most patients experience clearance of C. difficile with antibiotic therapy (metronidazole or vancomycin), approximately 20 % will experience another episode of CDI within 180 days of discontinuing antibiotics [31]. Multiple recurrence is seen in over half of patients who experience their first relapse and can range as high as 40–65 % in those previously treated for rCDI [31].
Recurrent CDI is thought to be the result of an inability of the intestinal microbiota to rehabilitate and re-establish itself after the initial antibiotic insult, thereby rendering the host susceptible to either a relapse with the same C. difficile strain or new re-infection with different strains of C. difficile [32, 33]. In the case of the former, a major mechanism for rCDI is believed to be C. difficile spore recrudescence or persistence from the initial infection.
It is interesting to note that not all individuals carrying C. difficile go on to develop symptomatic CDI [34, 35], suggesting that factors such as the microbiota and host immunity play an important role in preventing symptomatic infections. The healthy gut microbiota is dominated by the phyla Bacteroidetes or Firmicutes, which represent the evolutionary development of complementary yet distinct metabolic roles in the gut ecosystem [36]. Our knowledge of the microbial composition of the intestinal microbiota is evolving, largely due to technical advances in metagenomic analyses. Numerous studies have demonstrated decreased intestinal microbial diversity among patients with rCDI compared to those with only one episode of CDI that does not recur [33], and diversity is reduced in patients with primary or rCDI compared to those with asymptomatic colonization [33, 37, 38]. Other studies have shown lower proportions of the family Clostridiales Incertae Sedis XI in patients who developed CDI compared to those who did not [39–41]. This deviation in the gut microbiota is thought to contribute to the loss of colonization resistance to C. difficile, as seen in animal and in vitro studies [42]. Future endeavors to further profile the gut microbiome of individuals with asymptomatic CDI may unravel the dysbiotic mechanistic effects of C. difficile and identify specific microbial species that confer protection against CDI.
Risk factors for rCDI and ecosystem collapse of the healthy gut microbiota
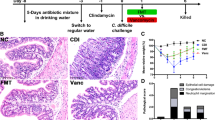
The indigenous intestinal microbiota is a highly dynamic ecosystem that is perturbed by environmental factors [38, 43, 44]. While the altered ecosystem seems to restore itself to homeostasis after CDI clearance with antibiotics, in the remaining 20 % of CDI cases, the microbiome has likely been altered beyond the point of spontaneous recovery, rendering the host susceptible to recurrent infection (Fig. 1).
Microbiome dysbiosis in recurrent C. difficile. The gut microbiome is a dynamic system, changing in structure and function when modulated by environmental factors. In recurrent C. difficile, various factors (1) contribute to the alterations and “imbalance” (dysbiosis) of the microbiome beyond spontaneous recovery, to a state incapable of clearing C. difficile infection. Instead, the altered microbiome creates a conducive environment for C. difficile colonization, sporulation, spore germination, and toxin production (2) C. difficile pathogenesis (through disruption of epithelial barrier function and alterations of inflammatory responses) likely contributes to the dysbiotic state (3) unless treated with microbiome therapy (4) to re-establish a more normal microbiome and to clear C. difficile infection (5). Blunt-head line indicates inhibitory effect. Shown in red are factors and effects contributing to recurrent C. difficile; shown in green are factors and effects important for clearance of recurrent C. difficile infection. PPIs proton pump inhibitors, FMT fecal microbiota transplantation, MET microbial ecosystem therapeutics, IL-8 interleukin-8, MCP1 monocyte chemotactic protein 1
Much of the evidence supporting the hypothesis that alteration of the microbiome is the primary determinant of recurrent CDI comes from associative studies rather than direct evidence implicating these factors. Thus far, widespread evidence has revealed that various factors predictive of rCDI—similar to those already mentioned for CDI—are suspected of microbiome modulation, including PPIs, antibiotic re-exposure after CDI treatment, fluoroquinolone use, appendectomy, surgery, chemotherapy, low immunoglobulin levels against C. difficile toxin A or B, age, and infection with hypervirulent B1/NAP1/ribotype 027 strains [45–51]. Other risk factors include the presence of an ileal pouch [52], fecal incontinence, and concomitant antacid use and hypoalbuminemia [47, 53–55]. For pediatric cases, recent evidence from Nicholson and colleagues showed that among 186 pediatric patients, malignancy (OR 3.39, 95 % CI 1.52–7.85), recent surgery (OR 2.40; 95 % CI 1.05–5.52), and the number of antibiotic exposures by class (OR 1.33, 95 % CI 1.01–1.75) were significantly associated with recurrent CDI [56]. Two of the above risk factors, PPIs and appendectomy, are explored in more detail below.
While repeated administration of antibiotics is a well-known risk factor, evidence is conflicting with regard to the association between CDI and exposure to proton pump inhibitors. Specifically, McDonald and colleagues found cause-specific hazard ratios for rCDI of 1.5 (95 % CI 1.1–2.0) for continuous PPI and 1.3 (95 % CI 0.9–1.7) for antibiotic re-exposure [45]. Deshpande et al. identified the use of PPI (RR 1.58; 95 % CI 1.13–2.21; P = .008) as the most frequent independent risk factor associated with rCDI, together with age greater than 65 years (RR 1.63; 95 % CI 1.24–2.14; P = .0005), additional antibiotics during follow-up (RR 1.76; 95 % CI 1.52–2.05; P < .00001), and renal insufficiency (RR 1.59; 95 % CI 1.14–2.23; P = .007) [57]. Not surprisingly, the risk for rCDI was also greater among patients previously on fluoroquinolones (RR 1.42; 95 % CI 1.28–1.57; P < .00001) [57]. While Linsky et al. found a 42 % increased risk of rCDI with PPI use during incident CDI treatment, a study by Freedberg et al. found no such association, instead noting a trend for reduced rCDI with PPI therapy, consistent with reports by Rotramel and colleagues [54, 58, 59]. Nevertheless, PPIs have been shown to affect the gastric microbiota [60], and a small pilot study recently found that PPI use decreased the diversity of the colonic microbiota [61]; as such, the effects of PPIs on the gut microbiome and rCDI warrant further investigation.
It is postulated that the appendix serves as a “safe house” for beneficial microbes, which re-inoculate the colon and serve as a reservoir for replenishment of the intestinal microbial ecosystem [62, 63]. This may explain the inverse association of the presence of the appendix with rCDI (adjusted RR 0.398; P < .0001). Specifically, Im et al. found a 2.5-fold increased risk of rCDI in patients without an appendix compared to those with an intact appendix [64]. This supports the suggestion that the presence of a vermiform appendix enhances recovery of a normal microbiome following CDI, thereby averting a relapse. Although a causal link is difficult to establish for most of these factors, in vivo/in vitro studies might prove useful for determining the role of the appendix in stabilizing a dysbiotic microbiota, the effect of immunoglobulins in the recovery of the protective microbiome, and differences in virulent strains, if any, on the composition and resilience of the gut microbiota.
Fecal microbiota transplantation (FMT)
Fecal microbiota transplantation (FMT) refers to the infusion of fecal suspension from a healthy donor to restore the gut microbiota of the recipient for therapeutic purposes. While the practice of FMT dates back to the Dong-jin dynasty in fourth century China, it was not until 1958 that Eiseman and colleagues first documented the use of fecal enemas as a treatment for antibiotic-induced pseudomembranous colitis in humans [65, 66]. Thereafter, Schwan et al. reported the use of FMT for recurrent CDI in a 65-year-old woman, whose symptoms promptly resolved within 24 h [67]. Over the past decade, there has been tremendous resurgence in the therapeutic role of FMT in a variety of clinical conditions, most notably in recurrent CDI.
Widespread evidence has shown FMT to be a safe, inexpensive, and efficacious treatment for recurrent CDI. Gough and colleagues, in their comprehensive review of FMT for recurrent CDI, found that 89 % of the 317 patients had resolution of recurrent CDI after one treatment. Furthermore, 87.5 % of the small percentage of patients who had relapsed CDI after the first FMT experienced clinical resolution with repeated treatments [68]. The robust systematic review by Kassam et al. compared 11 studies, reporting that up to 89.7 % of patients had resolution of CDI with FMT, with no adverse events in a follow-up period that ranged from weeks to years [69]. Cammarota et al., who included 20 case series, 15 case reports, and one randomized controlled trial in their systematic review, found that 87 % of a total of 536 patients experienced resolution of CDI-associated diarrhea, with no reported severe adverse events. The most common route of infusion was via colonoscopy, with a high response rate of 93 %. In the absence of head-to-head comparisons, the other routes—namely, nasogastric, nasoduodenal, or rectal enema—afford a comparative likelihood of cure, at 81, 86 and 84 %, respectively [70].
In 2013, van Nood et al. published the first randomized controlled trial of infusion of donor feces against rCDI, in a small open-label study using duodenal infusion. The patients were pretreated with vancomycin and bowel lavage and randomized to compare an initial high-dose oral vancomycin regimen (500 mg four times daily for 5 days) followed by bowel lavage and subsequent FMT, to a standard vancomycin regimen or a standard vancomycin regimen with bowel lavage. This trial was stopped early, after the interim analysis, since 94 % of patients in the FMT group achieved clinical resolution (81 % after the first infusion), compared to 31 or 23 % in the other treatment arms [71]. Likewise, Youngster et al. conducted an open-label randomized controlled pilot study with 20 patients with a median of four relapses and four antibiotic treatment failures, and found that FMT using a frozen inoculum from unrelated donors resulted in clinical resolution in 70 % of cases after a single treatment, which increased to 90 % with retreatment [72]. The monumental interest in fecal microbiota therapy is reflected in the number of trials listed on ClinicalTrials.gov with the keywords "Clostridium difficile" and "fecal microbiota transplantation," with at least 17 registered trials relevant to this topic currently underway in various phases [73].
FMT procedure
There have been various protocols across practitioners for donor eligibility, stool preparation. and administration. The donors are in good health and generally, but not always, known to the patient. While there is no established consensus, many guidance documents have been published [32, 74, 75]. Absolute contraindications usually include known or recent exposure to HIV or viral hepatitis, high-risk sexual behavior, past or present irritable bowel syndrome, inflammatory bowel disease, gastrointestinal malignancy, and recent antibiotic use. Both donors and recipients are screened for transmissible infectious pathogens, with serological testing for hepatitis A, B, and C, HIV, and syphilis, and stool tests for toxigenic C. difficile, ova and parasites, bacterial culture, and antibiotic sensitivity. Additional donor screening may include testing for vancomycin-resistant enterococci (VRE), methicillin-resistant Staphylococcus aureus (MRSA), carbapenem-resistant Enterobacteriaceae (CRE), Giardia, Cryptosporidium, and Isospora parasites, and enteric viral pathogens such as norovirus and rotavirus [76]. In cases of hematopoietic stem cell transplant and solid-organ transplant recipients, Epstein–Barr virus and Cytomegalovirus are also tested, and patients with relevant travel or exposure history may be tested for additional pathogens such as Strongyloides stercoralis. Donors are typically excluded if there has been antibiotic use within the last 3 months. More rigorous criteria excluding donors with chronic medical conditions such as obesity has also been recommended [76].
The processing of donor stool typically requires 50 g of stool to be re-suspended in approximately 100–300 mL of a variety of solutions ranging from the commonly used non-bacteriostatic saline, to water or milk. The re-suspension protocols vary from mixing in a beaker to homogenizing the solution in a mechanical blender followed by filtration of the larger particulate matter. Prior to administration, recipients usually undergo large bowel lavage and, in some protocols, are given anti-motility agents to optimize retention of fecal microbiota contents. FMT administered by any route has been shown to be efficacious, and there is no definitive evidence for the superiority of any one modality [68, 69, 74, 77]. Nevertheless, each modality has its inherent advantages and disadvantages. For instance, colonoscopy affords large volume infusions, visualization of comorbid pathology, and histologic evaluation, but entails a small risk of perforation. In comparison, nasoduodenal or rectal enemas are more accessible, less time-consuming, and cheaper.
Regulation
The widespread use of FMT and the concerns surrounding live microorganisms has led to the designation of stool as a biologic agent by Health Canada and by the Food and Drug Administration (FDA) in the United States. As such, the FDA requires an Investigational New Drug application and approval prior to the use of microbial-based therapeutics such as FMT. In July 2013, however, the FDA revised its requirements to exercise enforcement discretion in situations such as recurrent CDI while it develops appropriate policies about the use of FMT [78]. Health Canada followed suit with a similar guidance document release in March 2015 [79]. Inherent in the process of informed consent is the recipient's understanding of the investigational nature of this modality for treating C. difficile and its risks. Of note, the use of FMT for non-CDI indications still requires full regulatory approval in both the United States and Canada.
Safety
FMT is known to be a safe procedure, with adverse or serious complications reportedly uncommon [70]. Symptoms of constipation, diarrhea, cramping, and bloating have been reported shortly after FMT, but are transient and self-limiting [68]. Brandt et al., who conducted a multi-center long-term follow-up study of patients with rCDI previously treated with FMT, with a follow-up period ranging from 3 to 68 months, found a primary cure rate of 91 % among the 77 respondents. In addition, no definite adverse effects were noted, with 53 % of patients indicating a preference for FMT as first-line treatment if CDI were to recur [80]. To date, cases of death attributable to FMT due to aspiration pneumonia related to sedation and bacteremic toxic megacolon have been isolated, but underscore the need for robust follow-up to expand the safety data [81–83]. Although FMT has demonstrated overall safety among the immunocompromised population, a subset of patients with inflammatory bowel disease (IBD) have been found to experience exacerbation of IBD, fever, or elevated C-reactive protein levels post-FMT treatment [83–86]. The lack of data and the inherent variability of the microbial composition in donor stool renders the assessment of adverse events challenging. Nevertheless, further longitudinal data is absolutely imperative in establishing the safety of FMT, especially among select populations such as those with severe CDI and IBD.
Challenges of FMT
Although the multiple studies with FMT have provided a range of protocols, there are no protocols delineating definitive donor and recipient selection criteria, material processing, route of administration, or evaluation of success. A single standardized protocol may increase adoption rates across practitioners; thus far, Bakken et al. from the Fecal Microbiota Transplantation Workgroup have provided formal practice guidelines for FMT [74].
There are logistical and financial hurdles involved in conventional bacteriotherapy surrounding donor selection and screening, as well as concern for transmissible infections such as opportunistic pathogens or acquisition of infections in the limited time period prior to donation. While a healthy donor is essential in order to allow the restructuring of the recipient's microbiota to that of a healthy individual, the infectious risk is attenuated by the choice of donors known to the recipient based on the premise of shared commensal organisms. Nevertheless, there is adequate evidence that unrelated donor stool is just as efficacious [71, 87, 88]. Given the strict inclusion criteria, only a select proportion of recruited candidates are ultimately qualified. Khoruts et al., for example, found that approximately 10 % were qualified at their center [89]. The challenge of donor selection and recruitment could be mitigated by the development of dedicated donor programs with standardized screening and testing criteria that parallel blood donation programs.
Stool is an extremely complex material, consisting of a remarkably diverse range of microorganisms, with inter- and intra-individual variability in composition, representing the metabolically active and dynamic gut microbiota. Hence, a formidable challenge to conventional bacteriotherapy relates to stool processing that retains these inherent robust and resilient characteristics. Recent evidence has shown the feasibility of frozen microbial preparations [90, 91]. For instance, Youngster and colleagues demonstrated the efficacy of using frozen inoculum from unrelated donors as a treatment modality for relapsing CDI, with administration by nasogastric tube shown to be as efficacious as colonoscopic administration. As such, frozen inocula would eliminate the need for readily available donors and multiple donor screening and eligibility, thus facilitating the use of a single universal donor, expediting the process. Because it would bypass logistical hurdles through early identification and screening of donors ahead of time, it would allow these microbial fractions to become aesthetically appealing and quantifiable with respect to their bacterial composition, thereby enabling up-to-date testing of both donor and frozen product. Furthermore, banking of frozen microbial products would facilitate extended donor follow-up periods, thus permitting testing for latent infectious pathogens as well. While the mean shelf-life of the frozen inoculum has not been established, Youngster et al. reported a mean duration of 79.3 days, with a maximum length of 156 days. Harnessing this potential of frozen microbial fractions, non-profit stool banks such as OpenBiome in the United States supply standardized fecal microbiota for nasogastric or colonoscopic administration immediately after thawing or for storing up to 6 months at −20 °C. However, the FDA revision mandating that the FMT donor be known to either physician or recipient limits the full utilization of this service [92].
Among the widespread evidence for FMT in recurrent C. difficile cases, there is limited information about the nature of the bacterial species in donor stool samples or the intestinal microbiota in recipients. The gap in our knowledge of the microbial composition of the intestinal microbiota pre- and post-bacteriotherapy will slowly narrow with advances in metagenomic analysis. For instance, Khoruts et al. utilized 16S rRNA gene sequencing and terminal-restriction fragment length polymorphism analysis to demonstrate that clinical resolution after bacteriotherapy for rCDI was accompanied by the restoration of beneficial intestinal microbiota at 2 weeks post-transplantation. Specifically, the recipient colonic microflora resembled that of the donor and was dominated by Bacteroides species strains up to 33 days post-therapy. Furthermore, the Ruminococcaceae and Anaerostipes sp. strains became more abundant at later time periods [93]. Our evolving knowledge will eventually allow determination of the optimal composition of bacterial strains to restore gastrointestinal microbiota balance in treating recurrent CDI, and will facilitate safety and compliance in the administration of targeted microbiota-based therapeutics.
A major focus of research is the investigation of changes in intestinal microbiota species diversity and the underlying molecular mechanisms that confer resistance. Buffie et al. recently analyzed the intestinal microbiota of mice that were receiving antibiotics and went on to develop CDI, and compared this to the gut microbiota of bone marrow transplant patients who also received antibiotics, some of whom developed CDI. The authors found that a healthy intestinal microbiota comprises a large number of diverse species of microorganisms, with increased representation of Firmicutes and Bacteroidetes, and decreased representation of Proteobacteria. The authors further utilized mathematical modeling of gut microbiota composition data from mice and humans to identify bacterial species strongly associated with resistance to CDI, and then chose four species (Clostridium scindens, Blautia hansenii, Pseudoflavonifractor capillosus, and Barnesiella intestihominis), among which the strongest association was found for C. scindens. They then tested this mixture in their CDI animal model and found that administration of C. scindens either alone or as a mixture was protective, likely through mechanisms involving bile salt metabolism [94]. Although microbiome recovery appears to be critical in protecting against recurrent CDI, this study showed that specific key microbes are also likely important for protection against recurrent C. difficile infection.
Microbial ecosystem therapeutics
Microbial ecosystem therapeutics (MET) appears to be the natural progression to a more sophisticated approach to rehabilitating the gastrointestinal dysbiosis with a healthy ecosystem of native gut microbiota. Contrary to probiotic therapies, which comprise limited strains of bacteria, MET utilizes bacterial communities and is derived directly from the gastrointestinal tract. MET would be designed to account for a complex gastrointestinal microbiota, and thereby afford the recipient a means towards resilient and robust microbial communities.
Tvede and Rask-Madsen were early pioneers in identifying the need to move from fecal suspensions towards administration of defined and identified microorganisms as a therapeutic option. In 1989, the authors isolated ten enteric facultative aerobic and anaerobic bacterial strains from human feces, among which three of the selected strains inhibited the in vitro growth of C. difficile (R. productus, C. bifermentans, and an E. coli strain), whereas the six C. difficile strains inhibited the in vitro growth of all Bacteroides species [95]. Given that none of the six patients with recurrent CDI had this predominant colonic species in their gut prior to bacteriotherapy, and all had complete clinical recovery, with negative C. difficile toxin and evidence of sustained recolonization with Bacteroides sp. post-treatment, evidence at the time suggested that Bacteroides sp. was essential in normal colonic function and in colonic resistance to reinfection with C. difficile. Of note, one of the five patients had failed to improve with two fecal enemas, but did so with administration of this mixture of cultured bacteria [95].
The development of a prototype MET depends on a therapeutic formulation that optimally consists of various microbial species that represent the intricate stability and durability of the gut microbiota. For instance, Petrof et al. developed the "RePOOPulate" human-derived formulation consisting of 33 chosen isolates obtained from the stool of a single healthy donor. The authors used a modified continuous culture chemostat system to isolate the intestinal bacteria, and the 33 nonpathogenic strains were then chosen based on antibiotic susceptibility profiles, culturability, and stability of the ecosystem when grown in the continuous chemostat system [96]. This MET derived from a preselected ecosystem was administered via colonoscopy to two patients with recurrent CDI who had failed at least three courses of antibiotic therapy to treat CDI, resulting in clinical resolution that was persistent at the 6-month follow-up period. Additional 16S rRNA gene sequencing analysis demonstrated major changes in the stool microbial profile such that a minimum of 25 % of the RePOOPulate sequences were found in the recipient isolates up to 6 months post-treatment, despite incidental antibiotic perturbations, representing stability and feasibility of this treatment modality [96].
The use of advanced functional genomic and metabolomics technologies for the identification of microbes associated with protection, coupled with functional characterizations in murine model systems, will greatly benefit rational development of MET formulations [97]. For instance, Buffie et al. showed the protective effects of their single bacterial species C. scindens against CDI using a murine model. Likewise, Lawley et al. used mice infected with the epidemic C. difficile 027/B1, showing that it out-competed health-associated intestinal bacteria to perpetuate intestinal dysbiosis after clindamycin treatment. This murine model was then employed to rationally identify a simple mixture of six phylogenetically diverse bacteria that triggered the expansion of health-associated bacteria when administered as a mixture rather than as individual species, suggesting that both species composition and diversity are important factors in the design of bacteriotherapy [98]. These examples provide further proof of concept that targeted bacteriotherapy can cause robust shifts in microbial community structure, leading to disease resolution, and further support the rational approach of harnessing healthy microbial communities to treat diseases resulting from intestinal dysbiosis.
Advantages of MET
Cumulative evidence from the realm of “synthetic”, or defined, stool formulations as a therapeutic modality suggests that it will be an effective, feasible, and safe approach for the treatment of CDI. The concerns associated with fecal microbial therapy—notably, infection transmission, lack of standardized treatment regimens, and aesthetic issues—would be largely allayed with the use of synthetic stool substitutes.
Given the emerging evidence of the efficacy and feasibility of “probiotic” ecosystem therapeutics, it is worth elucidating its advantages over traditional simple-mixture probiotics or donor fecal administration. First, knowledge of the exact constituents of the synthetic stool substitutes enables protection from infectious pathogens, reproducibility, and control of the optimal composition that can then be recreated for further treatments at short notice. Second, synthetic probiotic mixtures represent a tailored treatment modality, since the profile of bacterial species can be selected based on specific criteria such as antimicrobial sensitivity patterns. Third, the formulation of a multi-species community derived from a selected ecosystem is more amenable to retaining the inherent characteristics of healthy gut microbiota necessary for robust colonization [99]. Furthermore, preparations of culture are more stable than fresh fecal suspensions, which are typically administered within 6 h of collection [74]. For instance, MET grown in continuous culture at steady state in a chemostat system would render defined microbial ecosystems readily available for use, enabling optimal establishment in the recipient. Petrof et al. have shown that the microbial composition of MET generated in their chemostat system at steady state was similar to the patient stool sample post-MET administration [100].
Challenges and future prospects
Conventional FMT bacteriotherapy and MET, by nature of their classification as biologic agents by the FDA and Health Canada, are subject to rigorous regulatory processes, some of which are unique to MET, given that the latter is technically a defined “manufactured” mixture. Nevertheless, the production, standardization, and regulation of microbial therapeutics will need to account for its complexity and variability. Good manufacturing practice (GMP) guidelines ensure that pharmaceutical manufacturing processes are consistent, and avoid contamination or product deviations that jeopardize safety and therapeutic efficacy. These principles can likely be enforced by regulatory agencies such as the FDA towards controlled production, design, monitoring, and control of manufacturing processes and facilities. Standardized practices such as these can safeguard optimal large-scale manufacturing once FMT-based therapeutics becomes mainstream. Thus far, the University of Minnesota has established GMP production of FMT therapeutics [99]. The establishment of large-scale manufacturing of microbiota therapeutics will entail significant capital investment for facilities and personnel that are ultimately equipped to consistently produce diverse, healthy, and robust mixtures of intact bacterial communities.
The concept of MET reflects the progression of “conventional” FMT to a more rationally designed treatment modality. Next-generation microbiota therapies, either as full-spectrum microbiota or synthetic defined MET, present an appealing opportunity to treat a range of chronic medical conditions wherein the human microbiota is thought to play a role, including inflammatory bowel disease, irritable bowel syndrome, obesity, and metabolic syndrome. This will ultimately be contingent upon an improved understanding of the mechanistic role of the intestinal microbiota in human physiology, both in health and disease.
Summary
Fecal microbiota transplantation and next-generation microbial-based therapeutics act to restore perturbed intestinal microbiota, and have shown tremendous potential in the treatment of recurrent CDI. This has since unleashed widespread interest in its use as a treatment modality for a range of challenging diseases. Conventional bacteriotherapy has led the way in the development of targeted microbial therapeutics based on our evolving understanding of the mechanisms by which microbial communities provide protection against certain diseases. Further research is needed on the long-term implications of microbiome manipulation.
References
Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65.
Hollister EB, Gao C, Versalovic J. Compositional and functional features of the gastrointestinal microbiome and their effects on human health. Gastroenterology. 2014;146(6):1449–58.
Bartlett JG, Gerding DN. Clinical recognition and diagnosis of Clostridium difficile infection. Clin Infect Dis. 2008;46(Suppl 1):S12–8.
George RH, Symonds JM, Dimock F, et al. Identification of Clostridium difficile as a cause of pseudomembranous colitis. Br Med J. 1978;1(6114):695.
Kelly CP, LaMont JT. Clostridium difficile–more difficult than ever. N Engl J Med. 2008;359(18):1932–40.
Hall I, O’Toole E. Intestinal flora in newborn infants with a description of a new pathogenic anaerobe. Bacillus difficilis. 1935;49:390–402.
Kwon JH, Olsen MA, Dubberke ER. The morbidity, mortality, and costs associated with Clostridium difficile infection. Infect Dis Clin North Am. 2015;29(1):123–34.
Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(Suppl 2):S88–92.
Burke KE, Lamont JT. Clostridium difficile infection: a worldwide disease. Gut liver. 2014;8(1):1–6.
Freeman J, Bauer MP, Baines SD, et al. The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev. 2010;23(3):529–49.
He M, Miyajima F, Roberts P, et al. Emergence and global spread of epidemic healthcare-associated Clostridium difficile. Nat Genet. 2013;45(1):109–13.
Khanna S, Pardi DS, Aronson SL, et al. Outcomes in community-acquired Clostridium difficile infection. Aliment Pharmacol Ther. 2012;35(5):613–8.
Centers for Disease Control and Prevention. (CDC). Severe Clostridium difficile-associated disease in populations previously at low risk-four states. MMWR Morb Mortal Wkly Rep. 2005;54(47):1201–5.
Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013;173(14):1359–67.
Ananthakrishnan AN. Clostridium difficile infection: epidemiology, risk factors and management. Nat Rev Gastroenterol Hepatol. 2011;8(1):17–26.
Wilcox MH, Mooney L, Bendall R, et al. A case-control study of community-associated Clostridium difficile infection. J Antimicrob Chemother. 2008;62(2):388–96.
Bolton RP, Tait SK, Dear PR, et al. Asymptomatic neonatal colonisation by Clostridium difficile. Arch Dis Child. 1984;59(5):466–72.
Tullus K, Aronsson B, Marcus S, et al. Intestinal colonization with Clostridium difficile in infants up to 18 months of age. Eur J Clin Microbiol Infect Dis. 1989;8(5):390–3.
Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec. Canada Clin Infect Dis. 2005;40(11):1591–7.
Johnson S. Recurrent Clostridium difficile infection: causality and therapeutic approaches. Int J Antimicrob Agents. 2009;33(Suppl 1):S33–6.
Bauer MP, Notermans DW, van Benthem BH, et al. Clostridium difficile infection in Europe: a hospital-based survey. Lancet. 2011;377(9759):63–73.
Garey KW, Dao-Tran TK, Jiang ZD, et al. A clinical risk index for Clostridium difficile infection in hospitalised patients receiving broad-spectrum antibiotics. J Hosp Infect. 2008;70(2):142–7.
Pépin J, Routhier S, Gagnon S, et al. Management and outcomes of a first recurrence of Clostridium difficile-associated disease in Quebec. Canada Clin Infect Dis. 2006;42(6):758–64.
Eyre DW, Walker AS, Wyllie D, et al. Predictors of first recurrence of Clostridium difficile infection: implications for initial management. Clin Infect Dis. 2012;55(Suppl 2):S77–87.
Hu MY, Katchar K, Kyne L, et al. Prospective derivation and validation of a clinical prediction rule for recurrent Clostridium difficile infection. Gastroenterology. 2009;136(4):1206–14.
Cohen MB. Clostridium difficile infections: emerging epidemiology and new treatments. J Pediatr Gastroenterol Nutr. 2009;48(Suppl 2):S63–5.
Kelsen JR, Kim J, Latta D, et al. Recurrence rate of Clostridium difficile infection in hospitalized pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17(1):50–5.
Rupnik M. Is Clostridium difficile-associated infection a potentially zoonotic and foodborne disease? Clin Microbiol Infect. 2007;13(5):457–9.
Keel K, Brazier JS, Post KW, et al. Prevalence of PCR ribotypes among Clostridium difficile isolates from pigs, calves, and other species. J Clin Microbiol. 2007;45(6):1963–4.
Bakken JS. Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe. 2009;15(6):285–9.
McFarland LV. Alternative treatments for Clostridium difficile disease: what really works? J Med Microbiol. 2005;54(Pt 2):101–11.
Allen-Vercoe E, Reid G, Viner N, et al. A Canadian working group report on fecal microbial therapy: microbial ecosystems therapeutics. Can J Gastroenterol. 2012;26(7):457–62.
Chang JY, Antonopoulos DA, Kalra A, et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197(3):435–8.
Kong LY, Dendukuri N, Schiller I, et al. Predictors of asymptomatic Clostridium difficile colonization on hospital admission. Am J Infect Control. 2015;43(3):248–53.
Alasmari F, Seiler SM, Hink T, et al. Prevalence and risk factors for asymptomatic Clostridium difficile carriage. Clin Infect Dis. 2014;59(2):216–22.
Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837–48.
Rousseau C, Poilane I, De Pontual L, et al. Clostridium difficile carriage in healthy infants in the community: a potential reservoir for pathogenic strains. Clin Infect Dis. 2012;55(9):1209–15.
Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4554–61.
Merrigan M, Sambol S, Johnson S, et al. Susceptibility of hamsters to human pathogenic Clostridium difficile strain B1 following clindamycin, ampicillin or ceftriaxone administration. Anaerobe. 2003;9(2):91–5.
Sambol SP, Merrigan MM, Tang JK, et al. Colonization for the prevention of Clostridium difficile disease in hamsters. J Infect Dis. 2002;186(12):1781–9.
Vincent C, Stephens DA, Loo VG, et al. Reductions in intestinal clostridiales precede the development of nosocomial Clostridium difficile infection. Microbiome. 2013;1(1):18.
Wilson KH. The microecology of Clostridium difficile. Clin Infect Dis. 1993;16(Suppl 4):S214–8.
Gerber GK. The dynamic microbiome. FEBS Lett. 2014;588(22):4131–9.
Caporaso JG, Lauber CL, Costello EK, et al. Moving pictures of the human microbiome. Genome Biol. 2011;12(5):R50.
McDonald EG, Milligan J, Frenette C, et al. Continuous proton pump inhibitor therapy and the associated risk of recurrent Clostridium difficile infection. JAMA Intern Med. 2015;5(175):784–91.
Fekety R, McFarland LV, Surawicz CM, et al. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin Infect Dis. 1997;24(3):324–33.
Garey KW, Sethi S, Yadav Y, et al. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect. 2008;70(4):298–304.
Cadena J, Thompson GR, Patterson JE, et al. Clinical predictors and risk factors for relapsing Clostridium difficile infection. Am J Med Sci. 2010;339(4):350–5.
Kyne L, Warny M, Qamar A, et al. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357(9251):189–93.
Leav BA, Blair B, Leney M, et al. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI). Vaccine. 2010;28(4):965–9.
Petrella LA, Sambol SP, Cheknis A, et al. Decreased cure and increased recurrence rates for Clostridium difficile infection caused by the epidemic C. difficile BI strain. Clin Infect Dis. 2012;55(3):351–7.
Seril DN, Ashburn JH, Lian L, et al. Risk factors and management of refractory or recurrent Clostridium difficile infection in ileal pouch patients. Inflamm Bowel Dis. 2014;20(12):2226–33.
Tal S, Gurevich A, Guller V, et al. Risk factors for recurrence of Clostridium difficile-associated diarrhea in the elderly. Scand J Infect Dis. 2002;34(8):594–7.
Rotramel A, Poritz LS, Messaris E, et al. PPI therapy and albumin are better predictors of recurrent Clostridium difficile colitis than choice of antibiotics. J Gastrointest Surg. 2012;16(12):2267–73.
Kim JW, Lee KL, Jeong JB, et al. Proton pump inhibitors as a risk factor for recurrence of Clostridium-difficile-associated diarrhea. World J Gastroenterol. 2010;16(28):3573–7.
Nicholson MR, Thomsen IP, Slaughter JC, et al. Novel risk factors for recurrent Clostridium difficile infection in children. J Pediatr Gastroenterol Nutr. 2015;60(1):18–22.
Deshpande A, Pasupuleti V, Thota P, et al. Risk factors for recurrent Clostridium difficile Infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36(4):452–60.
Linsky A, Gupta K, Lawler EV, et al. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med. 2010;170(9):772–8.
Freedberg DE, Salmasian H, Friedman C, et al. Proton pump inhibitors and risk for recurrent Clostridium difficile infection among inpatients. Am J Gastroenterol. 2013;108(11):1794–801.
Amir I, Konikoff FM, Oppenheim M, et al. Gastric microbiota is altered in oesophagitis and Barrett’s oesophagus and further modified by proton pump inhibitors. Environ Microbiol. 2014;16(9):2905–14.
Seto CT, Jeraldo P, Orenstein R, et al. Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome. 2014;2:42.
Bollinger RR, Barbas AS, Bush EL, et al. Biofilms in the normal human large bowel: fact rather than fiction. Gut. 2007;56(10):1481–2.
Guinane CM, Tadrous A, Fouhy F, et al. Microbial composition of human appendices from patients following appendectomy. MBio. 2013;4(1):e00366–12. http://mbio.asm.org/content/4/1/e00366-12.
Im GY, Modayil RJ, Lin CT, et al. The appendix may protect against Clostridium difficile recurrence. Clin Gastroenterol Hepatol. 2011;9(12):1072–7.
Zhang F, Luo W, Shi Y, et al. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol. 2012;11(107):1755 (author reply p-6).
Eiseman B, Silen W, Bascom GS, et al. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44(5):854–9.
Schwan A, Sjölin S, Trottestam U, et al. Relapsing Clostridium difficile enterocolitis cured by rectal infusion of homologous faeces. Lancet. 1983;2(8354):845.
Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53(10):994–1002.
Kassam Z, Lee CH, Yuan Y, et al. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108(4):500–8.
Cammarota G, Ianiro G, Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J Clin Gastroenterol. 2014;48(8):693–702.
van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–15.
Youngster I, Sauk J, Pindar C, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis. 2014;58(11):1515–22.
ClinicalTrials.gov. Search of “Clostridium difficile” and “fecal microbiota transplantation” studies 2015. https://clinicaltrials.gov/ct2/resultsterm=%22Clostridium+difficile%22+AND+%22fecal+microbiota+transplantation%22&Search=Search. Accessed 1 June 2015.
Bakken JS, Borody T, Brandt LJ, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9(12):1044–9.
Moayyedi P, Marshall JK, Yuan Y, et al. Canadian association of gastroenterology position statement: fecal microbiota transplant therapy. Can J Gastroenterol Hepatol. 2014;28(2):66–8.
Merenstein D, El-Nachef N, Lynch SV. Fecal microbial therapy: promises and pitfalls. J Pediatr Gastroenterol Nutr. 2014;59(2):157–61.
Silverman MS, Davis I, Pillai DR. Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol. 2010;8(5):471–3.
FDA Draft guidance for industry: enforcement policy regarding investigational new drug requirements for use of fecal microbiota for transplantation to treat Clostridium difficile infection not responsive to standard therapies. US. Department of Health and Human Services, Food and Drug Administration 2014. http://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/ucm387023.htm. Accessed 7 May 2015.
HealthCanada. Health Canada Guidance Document. Regulation of Fecal Microbiota Therapy for the treatment of C. difficile Infections 2015. http://www.hc-sc.gc.ca/dhp-mps/consultation/biolog/fecal_microbiota-bacterio_fecale-eng.php. Accessed 7 May 2015.
Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107(7):1079–87.
Solari PR, Fairchild PG, Noa LJ, et al. Tempered enthusiasm for fecal transplant. Clin Infect Dis. 2014;59(2):319.
Kling J Fecal Transplant an option even in the immunocompromised. October 16 2013. http://www.medscape.com/viewarticle/812685. Accessed 5 May 2015.
Kelly CR, Ihunnah C, Fischer M, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109(7):1065–71.
Angelberger S, Reinisch W, Makristathis A, et al. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol. 2013;108(10):1620–30.
De Leon LM, Watson JB, Kelly CR. Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol. 2013;11(8):1036–8.
Kump PK, Gröchenig HP, Lackner S, et al. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis. 2013;19(10):2155–65.
Hamilton MJ, Weingarden AR, Unno T, et al. High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria. Gut Microbes. 2013;4(2):125–35.
Shankar V, Hamilton MJ, Khoruts A, et al. Species and genus level resolution analysis of gut microbiota in Clostridium difficile patients following fecal microbiota transplantation. Microbiome. 2014;2:13.
Khoruts A, Sadowsky MJ, Hamilton MJ. Development of fecal microbiota transplantation suitable for mainstream medicine. Clin Gastroenterol Hepatol. 2015;13(2):246–50.
Hamilton MJ, Weingarden AR, Sadowsky MJ, et al. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107(5):761–7.
Youngster I, Russell GH, Pindar C, et al. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA. 2014;312(17):1772–8.
Rao K, Young VB. Fecal microbiota transplantation for the management of Clostridium difficile infection. Infect Dis Clin North Am. 2015;29(1):109–22.
Khoruts A, Dicksved J, Jansson JK, et al. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol. 2010;44(5):354–60.
Buffie CG, Bucci V, Stein RR, et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2015;517(7533):205–8.
Tvede M, Rask-Madsen J. Bacteriotherapy for chronic relapsing Clostridium difficile diarrhoea in six patients. Lancet. 1989;1(8648):1156–60.
Petrof EO, Gloor GB, Vanner SJ, et al. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome. 2013;1(1):3.
Rupnik M. Toward a true bacteriotherapy for Clostridium difficile infection. N Engl J Med. 2015;372(16):1566–8.
Lawley TD, Clare S, Walker AW, et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog. 2012;8(10):e1002995.
Petrof EO, Khoruts A. From stool transplants to next-generation microbiota therapeutics. Gastroenterology. 2014;146(6):1573–82.
Petrof EO, Claud EC, Gloor GB, et al. Microbial ecosystems therapeutics: a new paradigm in medicine? Benef Microbes. 2013;4(1):53–65.
Conflict of interest
Elaine O. Petrof is a co-founder and sits on the scientific advisory board of NuBiyota. Rowena Almeida and Teklu Gerbaba declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Almeida, R., Gerbaba, T. & Petrof, E.O. Recurrent Clostridium difficile infection and the microbiome. J Gastroenterol 51, 1–10 (2016). https://doi.org/10.1007/s00535-015-1099-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-015-1099-3