Abstract
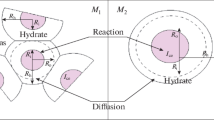
Assuming that gas hydrate formation from ice occurs through a diffusion mechanism, a system of equations was developed that describes the kinetics of gas hydrate formation from a spherical ice particle. This model takes into account the porous structure of the formed gas hydrate as well as the increases in particle volume due to the formation of the gas hydrate. In addition, the intrinsic kinetics of gas hydrate formation from ice is considered. In the framework of a quasi-stationary approximation, a simplified solution for the system of equations was obtained. From the comparison between the calculated and available experimental data, the temperature dependence was determined for the diffusion coefficient of methane in methane hydrate.



Similar content being viewed by others
References
Sloan ED, Koh CA (2008) Clathrate hydrates of natural gases, 3rd edn. CRS Press and Taylor & Francis Group, Boca Raton
Koh CA, Sum AK, Sloan ED (2009) Gas hydrates: unlocking the energy from icy cages. J Appl Phys 106:061101
Sloan ED (2003) Fundamental principles and applications of natural gas hydrates. Nature 426:353–359
Chatti I, Delahaye A, Fournaison L, Petitet J-P (2005) Benefits and drawbacks of clathrate hydrates: a review of their areas of interest. Energy Convers Manage 46:1333–1343
Mousis O, Lunine JI, Picaud S, Cordier D, Waite JH, Mandt KE (2011) Removal of Titan’s atmospheric noble gases by their sequestration in surface clathrates. Astrophys J Lett 740:L9
Gainey SR, Madden MEE (2012) Kinetics of methane clathrate formation and dissociation under Mars relevant conditions. Icarus 218:513–524
Marboeuf U, Schmitt B, Petit J-M, Mousis O, Fray N (2012) A cometary nucleus model taking into account all phase changes of water ice: amorphous, crystalline, and clathrate. Astron Astrophys 542:A82
Mousis O, Chassefière E, Lasue J, Chevrier V, Madden MEE, Lakhlifi A, Lunine JI, Montmessin F, Picaud S, Schmidt F, Swindle TD (2013) Volatile trapping in Martian clathrates. Space Sci Rev 174:213–250
Davidson DW, Garg SK, Gough SR, Handa YP, Ratclife CI, Ripmeester JA, Tse JS, Lawson WF (1986) Laboratory analysis of naturally occurring gas hydrate from sediment of the Gulf Mexico. Geochim Cosmochim Acta 50:619–623
Yakushev VS, Istomin VA (1992) Gas hydrate self-preservation effect. In: Maeno N, Hondoh T (eds) Physics and chemistry of ice. Hokkaido University Press, Sapporo, pp 136–140
Stern LA, Circone S, Kirby SH, Durham WB (2001) Anomalous preservation of pure methane hydrate at 1 atm. J Phys Chem B 105:1756–1762
Takeya S, Ebinuma T, Uchida T, Nagao J, Narita H (2002) Self-preservation effect and dissociation rates of CH4 hydrate. J Cryst Growth 237–239:379–382
Kuhs WF, Genov G, Staykova DK, Hansen T (2004) Ice perfection and onset of anomalous preservation of gas hydrates. Phys Chem Chem Phys 6:4917–4920
Ogienko AG, Kurnosov AV, Manakov AY, Larionov EG, Ancharov AI, Sheromov MA, Nesterov AN (2006) Gas hydrates of argon and methane synthesized at high pressures: composition, thermal expansion, and self-preservation. J Phys Chem B 110:2840–2846
Ohno H, Nishimura O, Suzuki K, Narita H, Nagao J (2011) Morphological and compositional characterization of self-preserved gas hydrates by low-vacuum scanning electron microscopy. ChemPhysChem 12:1661–1665
Sun D, Shimono Y, Takeya S, Ohmura R (2011) Preservation of carbon dioxide clathrate hydrate at temperatures below the water freezing point under atmospheric pressure. Ind Eng Chem Res 50:13854–13858
Nakoryakov VE, Misyura SYa (2013) The features of self-preservation for hydrate systems with methane. Chem Eng Sci 104:1–9
Mel’nikov VP, Nesterov AN, Reshetnikov AM (2003) Mechanism of gas hydrate decomposition at a pressure of 0.1 MPa. Dokl Earth Sci 389:455–458
Mel’nikov VP, Nesterov AN, Reshetnikov AM (2007) Formation of supercooled water upon dissociation of propane hydrates at T < 270 K. Dokl Phys Chem 417:304–307
Melnikov VP, Nesterov AN, Reshetnikov AM, Zavodovsky AG (2009) Evidence of liquid water formation during methane hydrates dissociation below the ice point. Chem Eng Sci 64:1160–1166
Melnikov VP, Nesterov AN, Reshetnikov AM, Istomin VA, Kwon VG (2010) Stability and growth of gas hydrates below the ice–hydrate–gas equilibrium line on the P-T phase diagram. Chem Eng Sci 65:906–914
Melnikov VP, Nesterov AN, Reshetnikov AM, Istomin VA (2011) Metastable states during dissociation of carbon dioxide hydrates below 273 K. Chem Eng Sci 66:73–77
Ohno H, Oyabu I, Iizuka Y, Hondoh T, Narita H, Nagao J (2011) Dissociation behavior of C2H6 hydrate at temperatures below the ice point: melting to liquid water followed by ice nucleation. J Phys Chem A 115:8889–8894
Vlasov VA, Zavodovsky AG, Madygulov MSh, Nesterov AN, Reshetnikov AM (2013) Pulsed NMR investigation of the supercooled water–gas hydrate–gas metastable equilibrium. Russ J Phys Chem A 87:1789–1792
Kuhs WF, Klapproth A, Gotthardt F, Techmer K, Heinrichs T (2000) The formation of meso- and macroporous gas hydrates. Geophys Res Lett 27:2929–2932
Staykova DK, Kuhs WF, Salamatin AN, Hansen T (2003) Formation of porous gas hydrates from ice powders: diffraction experiments and multistage model. J Phys Chem B 107:10299–10311
Kuhs WF, Staykova DK, Salamatin AN (2006) Formation of methane hydrate from polydisperse ice powders. J Phys Chem B 110:13283–13295
Takeya S, Hondoh T, Uchida T (2000) In situ observation of CO2 hydrate by X-ray diffraction. Ann N Y Acad Sci 912:973–982
Henning RW, Schultz AJ, Thieu V, Halpern Y (2000) Neutron diffraction studies of CO2 clathrate hydrate: formation from deuterated ice. J Phys Chem A 104:5066–5071
Halpern Y, Thieu V, Henning RW, Wang X, Schultz AJ (2001) Time-resolved in situ neutron diffraction studies of gas hydrate: transformation of structure II (sII) to structure I (sI). J Am Chem Soc 123:12826–12831
Wang X, Schultz AJ, Halpern Y (2002) Kinetics of methane hydrate formation from polycrystalline deuterated ice. J Phys Chem A 106:7304–7309
Kawamura T, Komai T, Yamamoto Y, Nagashima K, Ohga K, Higuchi K (2002) Growth kinetics of CO2 hydrate just below melting point of ice. J Cryst Growth 234:220–226
Rivera JJ, Janda KC (2012) Ice particle size and temperature dependence of the kinetics of propane clathrate hydrate formation. J Phys Chem C 116:19062–19072
Falenty A, Salamatin AN, Kuhs WF (2013) Kinetics of CO2-hydrate formation from ice powders: data summary and modeling extended to low temperatures. J Phys Chem C 117:8443–8457
Fujii K, Kondo W (1974) Kinetics of the hydration of tricalcium silicate. J Am Ceram Soc 57:492–497
Moudrakovski IL, Sanchez AA, Ratcliffe CI, Ripmeester JA (2001) Nucleation and growth of hydrates on ice surfaces: new insights from 129Xe NMR experiments with hyperpolarized xenon. J Phys Chem B 105:12338–12347
Vlasov VA (2013) Formation and dissociation of gas hydrate in terms of chemical kinetics. React Kinet Mech Catal 110:5–13
Cunningham RE, Williams RJJ (1980) Diffusion in gases and porous media. Plenum Press, New York
Kärger J, Ruthven DM (1992) Diffusion in zeolites and other microporous solids. Wiley, New York
Choi J-G, Do DD, Do HD (2001) Surface diffusion of adsorbed molecules in porous media: monolayer, multilayer, and capillary condensation regimes. Ind Eng Chem Res 40:4005–4031
Albo SE, Broadbelt LJ, Snurr RQ (2006) Multiscale modeling of transport and residence times in nanostructured membranes. AIChE J 52:3679–3687
Peters B, Zimmermann NER, Beckham GT, Tester JW, Trout BL (2008) Path sampling calculation of methane diffusivity in natural gas hydrates from a water-vacancy assisted mechanism. J Am Chem Soc 130:17342–17350
Acknowledgments
This work was supported by the grant of the President of the Russian Federation for leading scientific schools (No. NSh-3929.2014.5).
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: Interrelation between radii R(t) and ξ(t)
During the process of gas hydrate formation from ice, the following relations are satisfied:
where \(m_{\text{h}} (t)\) is the mass of the gas hydrate, \(m^{\prime}_{\text{g}} (t)\) is the mass of the gas passed into the structure of the gas hydrate, \(n^{\prime}_{\text{g}}\) is the moles of the gas passed into the structure of the gas hydrate and \(n^{\prime}_{\text{w}}\) is the moles of water passed into the structure of the gas hydrate. From Eqs. (30)–(32), it follows that
Substituting Eq. (33) into Eq. (26) yields
The geometry of the problem allows us to represent the quantities \(m_{\text{h}} (t)\) and \(m_{{{\text{i}}0}}\) in the form
where \(\rho^{\prime}_{\text{h}}\) is the apparent mass density of the gas hydrate and \(\rho_{\text{h}}\) and \(\rho_{\text{i}}\) are the true mass density of the gas hydrate and of ice, respectively. Substituting Eqs. (35) and (36) into Eq. (34), we can obtain the relation
Taking into account Eq. (27) and given that \({ \rho}_{\text{i}} = \omega M_{\text{w}}\) and \(\rho_{\text{h}} = \chi M_{\text{h}}\), Eq. (37) goes into Eq. (12).
Appendix 2: Equation of motion for the front of reaction (1)
Near the surface Γ, the following molar balance ratio for gas hydrate is satisfied:
From Eq. (38), taking into account the geometry of the problem, we can obtain the equation of motion for the front reaction (1) in the form
With allowance for Eq. (7), Eq. (39) goes into Eq. (13).
Near the surface Γ, the following molar balance ratio for water is also satisfied:
Taking into account the geometry of the problem and given Eq. (6), from Eq. (40) the equation of motion for the front reaction (1) can be obtained in the form
Thus, the equation of motion for the front reaction (1) is written ambiguously. However, analysis shows that this has little effect on the results of simulation for the kinetics of gas hydrate formation from ice in the framework developed by the diffusion model.
Rights and permissions
About this article
Cite this article
Vlasov, V.A. Diffusion model of gas hydrate formation from ice. Heat Mass Transfer 52, 531–537 (2016). https://doi.org/10.1007/s00231-015-1575-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-015-1575-6