Abstract
Renal interstitial fibrosis and tubular atrophy (IFTA) is one of the primary end points of kidney injury, and accurate IFTA quantitation in biopsy samples is crucial in establishing the diagnosis and assessing disease severity. Therefore, knowing the basic procedures in the preparation of biopsy for IFTA and specific staining techniques available for IFTA is pivotal to the pathologists’ practice. This chapter reviews the mechanisms of IFTA pathogenesis pertinent to biopsy evaluation and the common techniques used to evaluate biopsies for IFTA. The challenges facing IFTA evaluation in biopsies and recent technical developments in this field are discussed.
Similar content being viewed by others
Keywords
- Kidney/renal fibrosis
- Epithelial–mesenchymal transition
- Myofibroblast
- Morphometry
- Masson’s trichrome stain
- Picrosirius red stain
Definitions
Birefringent
Birefringence in the context of this article refers to colors that are somewhat iridescent or anomalous (Howie et al. 2008). This anomalous quality is imparted to tissue when viewed under polarized light due to a particular interaction of the polarized light with the tissue or a combination of the stain’s interaction with the tissue. For example, a Sirius red stain of fibrotic tissue, particularly the collagen in this fibrotic tissue, has a yellowish birefringence when viewed under polarized light.
Computerized morphometry
Tissue is imaged and then analyzed with computerized programs called algorithms to obtain quantitative measures of different parameters. For example, a positive pixel count algorithm tuned to detect the color of fibrotic tissue can be used to quantitate fibrotic areas.
Endothelial/mesenchymal transition (EMT)
The change in endothelial phenotype during renal injury leading to fibrosis, in which endothelial cells lose their specific markers and acquire the mesenchymal phenotype, becoming more invasive with increased migratory abilities.
Epithelial/mesenchymal phenotype (EMP)
The putative phenotypic change of epithelial cells leading to the loss of their cell polarity and adhesion surface proteins and gain of migratory properties to become mesenchymal stem cells.
Extracellular matrix (ECM)
Connective tissue material that essentially serves as the framework for tissue and that is not contained within cells.
Fibrosis
This term refers to the deposition of excess fibrous tissue within an organ and is derived from the Latin fibra, meaning “fiber,” and the Greek -osis, meaning a condition or state [derived from Dictionary.com].
Genomics
The analysis of the genetic state of a biologic specimen or set of biologic specimens. Genomics is one of the main “-omics” disciplines in which the suffix “-ics” derived from the Latin “-ica” and Greek “-ika” [derived from Dictionary.com].
IFTA
Parenchymal contraction in which intervening tubulointerstitial parenchyma between obsolescent glomeruli have been lost and occupied by fibrotic matrix through interstitial fibrosis (IF), which is usually accompanied by tubular atrophy (TA).
microRNA
Short single-stranded RNA molecules that regulate a wide range of genes posttranscriptionally by targeting mRNAs and are stable in tissues for extending periods of time.
Proteomics
Large-scale study of structures and functions of proteins using protein purification techniques and mass spectrometry. Referring to proteome as the entire set of proteins of an organism or tissue. This is an example of another “-omics” discipline.
Sclerosis
Refers to hardening or stiffening of tissue or organ, often due to a pathologic growth of fibrous tissue or chronic inflammation [derived from Dictionary.com]. The word is derived from the Greek sclerosis, which means “hardening.”
Introduction
Interstitial extracellular matrix (ECM) accumulation is common to many chronic kidney diseases and contributes to loss of kidney function. After renal damage, a constellation of intra- and extrarenal cells are activated to limit the extent of tissue damage and start the healing process. Interstitial fibrosis is the result of such processes (Table 1), when a complete regeneration of the renal tissue cannot be achieved. Interstitial fibrosis (IF) mostly refers to the excessive and pathological deposition of ECM, which is often accompanied by tubular atrophy (TA) and is collectively termed IFTA (Liu 2006; Boor et al. 2010; Zeisberg and Neilson 2010; Farris and Colvin 2012; Farris and Alpers 2014). TA is characterized by the presence of small dilated and thin tubules with pale cytoplasm and thickened and irregularly contoured basement membranes. TA probably has distinct mechanisms than IF, related to blood flow restriction, glomerular filtration rate (GFR), or loss of tubular continuity. Many studies show a reciprocal correlation between kidney function and the IF extent. IF has been shown to predict the outcome of renal allograft (Choi et al. 2005; Farris et al. 2010; Meas-Yedid et al. 2011) and native kidney diseases such as IgA nephropathy (Working Group of the International IgA Nephropathy Network and the Renal Pathology Society et al. 2009).
Renal biopsies play major role in clinical diagnosis of IFTA. However, there is still controversy over the best qualitative and quantitative techniques for IFTA (Farris et al. 2014). Accurate IFTA measurement is required in numerous applications, including in comparison of renal allograft protocol biopsies, helping in the determination of prognosis in glomerular disease (e.g., IgA nephropathy and lupus nephritis), and pharmaceutical studies on therapeutic inhibition of IF (Liu 2006; Vilayur and Harris 2009). Furthermore, as stem cell therapy is gaining attention in renal fibrosis research, the need for establishing accurate and valid assessment techniques for fibrosis is more pronounced (Reinders et al. 2014; Choi et al. 2015).
Mechanisms of Renal Fibrosis
Normal renal interstitium consists of sparse cells, mainly fibroblasts and dendritic cells (DCs), embedded in ECM network. During and after renal injury (infection, ischemia, diabetes, allograft rejection, etc.), a constellation of mechanisms are activated to protect the damaged tissue and speed up regeneration (such as reepithelialization, epithelial barrier repair, regeneration after vascular injury, construction of the ECM skeleton, and recovering from mesangial damage). A variety of cells (Fig. 1) and molecular mechanisms (Fig. 2) contribute to IF.
Cells contributing to interstitial fibrosis: A variety of cells contribute to interstitial fibrosis, including renal vasculature, tubular cells, and inflammatory cells such as lymphocytes, monocyte/macrophages, mast cells, and dendritic cells. The renal tubules undergo changes to acquire epithelial–mesenchymal phenotype (EMP) and may undergo the process of epithelial–mesenchymal transition (EMT) . The endothelium is possibly involved in a process of endothelial–mesenchymal transition (EndoMT). Inflammatory cells have important role in both the processes of EMT/EMP and EndoMT. Fibrocytes and pericytes can transform to fibroblasts and myofibroblasts . Fibroblasts/mesenchymal cells mediate the production of fibrosis and extracellular matrix (ECM) deposition and also may undergo a transition to a myofibroblastic phenotype through paracrine signaling from inflammatory cells, further increasing ECM deposition and fibrosis
Key molecular mechanisms contributing to interstitial fibrosis: A variety of molecular mechanisms contribute to interstitial fibrosis. Transforming growth factor (TGF-β) is released from activated inflammatory and damaged mesangial cells through interactions with matrix metalloproteinases (MMPs), plasmin, integrin, angiotensin, tissue plasminogen activator (tPA), and tissue transglutaminase (tTG). When it is released from inhibition by latent TGF-β-binding protein (LTBP) and latency-associated peptide (LAP), TGF-β binds the transforming growth factor receptor (TGF-R), activating intracellular signals such as the Smads, jagged/notch, Akt, Bcl-2, and NF-κB pathways. These lead to nuclear transcription, ultimately culminating in collagen and ECM production. In epithelial cells it may lead to epithelial-mesenchymal transition (EMT) . Smads also increase fibrosis via integrin-linked kinase (ILK). ILK acts through glycogen synthase kinase (GSK) to produce β-catenin, which traverses into the nucleus to induce transcription. Integrins can also act through ILK in a similar manner. Bone morphogenic proteins (BMPs) generally restrict fibrosis. BMPs bind to their receptor (BMPR) to inhibit Smads, a process inhibited by uterine sensitization-associated gene 1 (USAG-1) and platelet-derived growth factor (PDGF)
IF is most likely driven by a lack of highly differentiated cells, which are replaced by scarring connective tissue. Tumor growth factor beta (TGF-β) is a major fibrogenic and inflammatory cytokine, produced by damaged native and inflammatory cells (Farris and Colvin 2012; Friedman et al. 2013). Tubular epithelial cells unable to regenerate get arrested in the G2/M phase and produce TGF-β (Yang et al. 2010), which causes augmented deposition of ECM proteins and renal fibrosis (Bottinger 2007). TGF-β-inducible integrins (e.g., αVβ6) ultimately act through integrin-linked kinases (ILK) and other mediators to produce collagen, contributing to the production of the ECM (Farris and Colvin 2012). ECM production is also affected by other systemic physiologic states, such as the renin/angiotensin system (Naito et al. 2010).
ECM accumulation usually occurs prior to chronic kidney disease (CKD) and contains sulfated and non-sulfated proteoglycans and glycosaminoglycans involved in IF and other major molecules such as types I and III collagen, fibronectin, biglycan, decorin (Boor et al. 2010; Zeisberg and Neilson 2010), tissue transglutaminase (tTG) (Huang et al. 2009), matrix metalloproteinase (MMP) (Wang et al. 2010), tissue plasminogen activator (tPA) (Yang et al. 2002), and laminin (Abrass et al. 2010). Proteoglycans fill the majority of renal extracellular interstitial space and act as a reservoir of pro-fibrotic growth factors, such as the latent forms of TGF-β or fibroblast growth factor 2 (FGF-2). Fibronectin and thrombospondin-1 (TSP-1) are adhesive glycoproteins involved in IF. Fibronectin accumulation is one of the first events during renal fibrosis (Eddy 1996).
Other mediators important in IF include Smads, bone morphogenic proteins (BMPs), particularly BMP-7, sclerostin domain-containing protein 1 (also known as uterine sensitization-associated gene 1 (USAG-1)), protein kinase C (PKC), extracellular-signal-regulated kinases (ERKs) (Sun et al. 2014), platelet-derived growth factor (PDGF), PDGF-β (Wilkinson et al. 2009), hepatocyte growth factor (HGF), the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway, fibrinogen (Sorensen et al. 2011), and possibly toll-like receptors (TLRs). Smads act on ILK, stimulating glycogen synthase kinase (GSK) to produce β-catenin, which traverses into the nucleus to induce transcription that ultimately leads to fibrosis (Farris and Colvin 2012).
Renal fibroblasts are the major constituent cells in ECM and are in charge of producing excessive collagen (Boor et al. 2010). Thrombospondin-1 stimulates fibroblast proliferation and migration in CKD and is correlated with the degree of tubulointerstitial fibrosis in rat models of renal fibrosis (Mason and Wahab 2003). Fibroblasts stain for vimentin (intermediate filament protein) and stain weakly for alpha smooth muscle actin (α-SMA). Activated fibroblasts stain for fibroblast-specific protein 1 (FSP-1). However, there are not any completely specific markers for fibroblasts that are widely utilized, and this makes the study of fibroblasts quite difficult (Xia et al. 2013; Farris and Alpers 2014).
Myofibroblasts secrete collagen and glycosaminoglycans and are activated by various mechanisms such as paracrine signals derived from lymphocytes and macrophages, autocrine factors, and pathogen-associated molecular patterns (PAMPS). Myofibroblasts have multiple potential origins with candidates being fibroblasts, fibrocytes, pericytes, and epithelial or endothelial cells (Humphreys et al. 2010). These cells share features with smooth muscle cells, including the expression of α-SMA, and contain vimentin, fibronectin, and S100A4 (also known as FSP-1) (Lin et al. 2008). Pericytes contribute to vascular reconstruction via tissue inhibitors of metalloproteinases (TIMPs) and ADAMTS1 (Schrimpf et al. 2012). Fibrocytes are derived from peripheral blood leukocytes (Pilling et al. 2009) producing ECM and expressing both hematopoietic (e.g., CD45) and stromal cell markers (e.g., type I collagen). Recent in vitro studies suggest that fibrocytes develop outside the kidney independent of infiltrating monocytes and rely on CCR2 for migration into target organs (Reich et al. 2013).
Endothelial cells and tubular epithelial cells (TECs) participate in the recruitment of circulating leukocyte populations and facilitating the inflammatory response in the injured kidney. TECs play major role in recruiting macrophages and lymphocytes via NF-κB and pro-inflammatory chemokines (Mezzano et al. 2004). Epithelial and endothelial cells increase the IF through a process of differentiation to myofibroblasts , in which they undergo a phenotypic conversion termed epithelial–mesenchymal or endothelial–mesenchymal transition (EMT/EndoMT) (LeBleu et al. 2013). In this process they lose their markers, such as E-cadherin, and acquire mesenchymal markers, such as vimentin and α-SMA (Zeisberg and Neilson 2010; Friedman et al. 2013). These markers can be used to determine EMT process in the tissue (Wang et al. 2015). However, many experts have questioned this migration feature of EMT (Kriz et al. 2011). Endothelial cells can suppress inflammation via endothelial nitric oxide synthase (eNOS) production in injured sites. VEGF alleviates fibrosis (Lian et al. 2011), and hypoxia promotes fibrosis through multiple mediators including hypoxia-inducible factor-1α (HIF-1α) (Higgins et al. 2008).
A wide range of mononuclear inflammatory cells can be identified in normal renal interstitium, including DCs, macrophages, and lymphocytes (T cells, B cells, and natural killer cells) (Paust et al. 2011). Lymphocytes play a variety of roles in the development of IFTA (Tapmeier et al. 2010). Type 2 T-helper cells (TH2) produce mostly pro-fibrotic cytokines, inducing differentiation of fibrocytes, triggering macrophage recruitment and inflammatory response (Liu et al. 2012), and in contrast, type 1 T-helper cells (TH1) inhibit differentiation of fibrocytes (Niedermeier et al. 2009). Microarray analysis of renal allografts has shown increased T cell and natural killer gene sets in IFTA development (Scian et al. 2011). High T cell and macrophage but not B cell infiltration is associated with low IL-10 expression, which confers susceptibility to IFTA (Khan et al. 2010).
Monocyte/macrophages are heterogeneous, consisting of both infiltrating and resident cells (Anders and Ryu 2011). Renal DCs seem to mediate the recruitment of other cell types. During exposure to bacteria, DCs generate chemokines to attract effector cells, such as neutrophils (Rogers et al. 2014). Macrophages or DCs can be identified by F4/80 (also known as EGF-like module-containing mucin-like hormone receptor-like 1 (EMR1), CD11b+ (integrin αM), and the DC marker CD11c+ (integrin αx). However, these markers are not specific (Rogers et al. 2014). The degree of macrophage infiltration correlates with both the severity of damage and extent of IF (Eardley et al. 2008). Some subsets of bone marrow-derived monocytes such as CD11b+ cells may attenuate fibrosis (Semedo et al. 2010).
Mast cells express immune-related surface receptors and store inflammatory cytokines, which give them the ability to immediately release pro-fibrosis mediators such as TGF-β and MMPs (Snelgrove et al. 2011). There is a correlation between the accumulation of mast cells and the degree of renal interstitial fibrosis (Mack and Rosenkranz 2009).
Interstitial Fibrosis Assessment
Interstitial fibrosis is typically assessed in a qualitative and a quantitative manner. A variety of methods can be used (Fig. 3) and are discussed below. Briefly, microscopy is one of the primary methods of this assessment since it assists in the qualitative measurement of fibrosis, and it also allows quantitation of the degree of fibrosis. Furthermore, molecular methods are becoming more widespread and will also likely aid in both qualitative and quantitative assessment of fibrosis (Table 2).
Methods for interstitial fibrosis assessment: Various methods are available for assessing interstitial fibrosis. Renal biopsy samples are routinely prepared for light, immunofluorescent, and electron microscopic studies. For clinical evaluation of renal fibrosis, H&E, trichrome, Periodic acid–Schiff (PAS), picrosirius red, and collagen III immunohistochemical (IHC) stainings are commonly used. Other methods have been used for research only purposes to study renal fibrosis, such as electron microscopy, microRNA analysis, or transcriptomics or proteomics. However, these methods are not yet used for routine clinical evaluation of renal fibrosis
Morphologic approaches for interstitial fibrosis assessment: Interstitial fibrosis can be approached in two basic ways by pathologists. (1) The fibrosis percentage taken as the percentage of tissue occupied by fibrosis excluding tubules and glomeruli and healthy islands of tissue, (2) assessing the percentage of the tissue that is abnormal
Fibrosis Patterns in Biopsies
It is important to note that depending on the pathology there are different patterns of IFTA. Table 3 highlights the features of IFTA in different pathologic conditions.
Despite these associations, there is often an essentially nonspecific pattern of fibrosis in renal biopsies of patients with chronic kidney disease, including diffuse or patchy fine IF surrounding tubules, which can be either normal or atrophic. This is associated with either diffuse or focal disease of glomeruli, tubules, or vessels (Farris and Colvin 2012; Farris and Alpers 2014; Haas et al. 2014). It is important to note that even though the clinical emphasis is usually put on cortical IF, medullary IF is likely happening in parallel to cortical IF and epithelial loss (Farris et al. 2013).
Staining Techniques and Light Microscopy
The preferred approach in staining for IFTA is using multiple complementary techniques to confirm the degree of fibrosis, because each technique has shortcomings and advantages. The routine techniques to obtain sample and assess renal fibrosis and tubular atrophy may be different between laboratories. Hence, a generally accepted algorithm is usually applied in each laboratory (Agarwal et al. 2013).
In the assessment of IFTA, paraffin embedded kidney sections are often stained for hematoxylin and eosin (H&E), trichrome, periodic acid–Schiff (PAS), and methenamine silver. Other methods such as picrosirius red or immunohistochemistry for collagen (particularly collagen types I and III) or SMA can give more information depending on the specimen. Table 4 summarizes the characteristics, advantages, and disadvantages of currently popular stains for IFTA in routine clinical diagnosis and research. Trichrome highlights fibrosis by color, typically with a blue or green hue, depending on the trichrome method employed (e.g., Mallory’s, Masson’s, etc.). For quantitation, visual assessment of slides is the standard practice at many institutions (Moreso et al. 2001). Picrosirius red stain is also a commonly used method to detect fibrosis in tissues (Fig. 5). Picrosirius red is typically considered to be specific for collagen types I and III under polarized light in which the collagen has a birefringent yellow hue (Sund et al. 2004). Movat’s pentachrome stain allows the assessment of collagen, proteoglycan, and elastic tissue content with a single-staining procedure. It imparts different colors to collagen fibers, glycosaminoglycans, elastic fiber, fibrin, nuclei, and muscle. There have been modifications for this staining to increase consistency and reliability and decrease preparation time (Doello 2014). Immunohistochemistry (IHC) staining can be used to identify particular protein or cell population in the biopsy sample, such as collagen I, III, and IV, smooth muscle actin (e.g., α-SMA) (Choi et al. 2015), Smad7, E-cadherin (Liu et al. 2013), and CD11c+ cells (Kruger et al. 2004). Among the wide range of IHC stains available, collagen III (Satoh et al. 2001) is most often used to assess fibrosis (Fig. 5).
Commonly used stains to assess renal fibrosis are shown: Fibrosis is visualized by different colors in each staining: Blue in trichrome, light pink/purple in PAS (vs. dark pink/purple for basement membranes), dark brown in collagen III immunohistochemistry, and red in picrosirius red stain . A glomerulus can be seen in the center of the images, and an area of fibrosis can be seen to the left of the glomerulus in each of the images (all at an original magnification of 200×)
Computer-Based Morphometric Study
Morphometry techniques use computerized image analysis to measure the surface area affected by fibrosis. These computer-based methods include morphometry of slides stained with trichrome (Farris et al. 2011, 2014; Meas-Yedid et al. 2011), picrosirius red (Sund et al. 2004; Lattouf et al. 2014), and collagen III IHC (Farris et al. 2011, 2014; Farris and Colvin 2012) or a combination of staining techniques and automated analysis (Street et al. 2014). Analysis in some of these studies has shown correlation with GFR (Farris et al. 2011, 2014; Meas-Yedid et al. 2011); however, as shown in the studies by Farris et al. (2011), this may not improve upon the assessment made by the unaided human eye (Farris et al. 2011). As computerized scanners gain more widespread use in research and clinical settings, it is possible that computerized morphometry will integrate more in clinical standard practice. Commonly used software programs include NIH ImageJ (available at http://imagej.nih.gov/ij/), Fiji (available at http://fiji.sc/Fiji), Icy (available at http://icy.bioimageanalysis.org/), and proprietary programs that are stand-alone and/or are provided by microscope manufacturers (e.g., Aperio [Leica], Olympus, NIS-Elements [Nikon], MetaMorph, etc.). Whole slide scanners allow scanning of slides, often in an automated batch mode, which further facilitates the ease of this analysis (Farris et al. 2011, 2014).
Molecular Methods
A variety of molecular pathways can be probed through transcriptional profiling of gene expression (Maluf et al. 2008; Bunnag et al. 2009; Scian et al. 2011). Many approaches involve examining genes from set pathways together to determine which pathways predominate in IFTA, showing, for example, the importance of chronic inflammation in a study that examined immune response, cell-to-cell interaction, and inflammation pathways (Maluf et al. 2008). Other studies show the importance of specific derangements in the development of IFTA, showing that tissue injury and dedifferentiation are linked with functional deterioration (Bunnag et al. 2009). Older studies have focused on the analysis of DNA and RNA; however, newer studies have evaluated microRNAs (miRNAs), short single-stranded RNA molecules that regulate wide ranges of genes posttranscriptionally by targeting mRNAs (Zununi Vahed et al. 2014). MicroRNAs are stable in tissues for extended periods of time. So far a few miRNAs are the center of clinical attention, which may find implications in renal fibrosis assessment, especially in renal allograft tissue (Zununi Vahed et al. 2014; Chung and Lan 2015) with some miRNAs being pro-fibrotic and upregulated in renal fibrotic tissue and some miRNAs showing a decrease in IFTA (miR-107, 211, 204, 324, and 30a-3p) (Chung and Lan 2015).
Proteomic Approaches, Including Mass Spectrometry
There are a growing number of studies on fibrotic pathophysiological mechanisms using proteomic approaches in cell cultures, animal models, and human tissues (Klein et al. 2011; Prunotto et al. 2011). Many proteomic methods utilize techniques for protein fractionation and mass spectrometry (Konvalinka et al. 2010; Sethi et al. 2013). Proteomic methods can potentially be used on a variety of specimens, including urine, to provide noninvasive assessment of renal fibrosis. Current proteomic work has mainly explored in vitro systems. Databases, specific for normal and pathological human kidney proteomes, are available including normal glomeruli and medulla (e.g., http://www.hkupp.org/ and http://www.proteinatlas.org/humanproteome/kidney) (Habuka et al. 2014). However, despite the recent technical advancements and studies, proteomic biomarkers have not yet found direct implications in clinic (Konvalinka et al. 2010; Sethi et al. 2013).
Current techniques have specific shortcomings when it comes to IFTA evaluation as the whole kidney proteomic analysis does not provide accurate information regarding localization (Walker et al. 2004), and, therefore, each kidney compartment should be separated prior to proteomic analysis (such as with laser capture microdissection). Still, the minute amount of available tissue severely limits exploration.
Fibrosis Measurement and Methodologies: Challenges and Promises
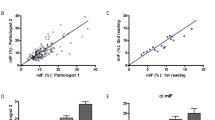
The measurement of IF has limitations including sampling issues and variable quality and quantity of the IF. Overall, there is no consensus regarding the best way to assess IF (Farris et al. 2014; Haas et al. 2014). Unfortunately, there is a lack of consistency in measuring fibrosis among pathologists, which stems from different conceptual ways of considering the percentage of fibrosis. Some pathologists consider IF percentage as the percent of overall tissue occupied by fibrous matrix, whereas others consider the area containing both fibrotic matrix and intact glomeruli and tubular structures. This is depicted in the Fig. 4. This discrepancy was highlighted in an interobserver variability study among numerous pathologists in multiple countries by Furness et al. (2003), and a recent study prompted by the Banff Allograft Pathology Conference also showed a great deal of variability in fibrosis assessment among pathologists (Farris et al. 2014). Discrepancies among pathologists involve the threshold of matrix needed in order to identify a region as being involved by fibrosis. Therefore, special effort should be made to overcome this caveat by constant education and unifying the definition.
Potential Applications to Prognosis and Other Diseases or Conditions
Fibrosis assessment is important in the kidney and also other organs because it provides a surrogate marker of chronic injury. IFTA assessment is important in the kidney in particular because it shows correlations with renal function (Farris et al. 2014; Farris and Alpers 2014). Some investigators distinguish conventional fibrosis from “sclerosis” since sclerosis may represent a late “hardened” stage at which fibrosis is more chronic in nature and thus be pathologically distinct. It is likely that future method refinements will help recognize later stages of fibrosis and also identify stages at which fibrosis may be reversible (Farris and Colvin 2012; Farris and Alpers 2014), and intervention may be possible at earlier stages of fibrosis (Friedman et al. 2013). Furthermore, fibrosis assessment is useful in a variety of other diseases including pulmonary fibrosis, liver fibrosis (cirrhosis), nephrogenic systemic fibrosis, systemic sclerosis, wound healing, and cancer (Farris and Colvin 2012; Friedman et al. 2013; Farris and Alpers 2014; Rybinski et al. 2014). Therefore, although this review has focused mostly on the kidney, it is likely that the kidney can serve as a window to view other organs and disease processes and thus improve the lives for patients (Friedman et al. 2013).
Conclusion
IFTA is brought about through complex molecular mechanisms of renal injury. Fibrosis can be assessed in a number of ways. Efforts to improve these methods could lead to improvements in the surrogate measures provided by pathologists, making pathologic assessment a better biomarker of renal disease, and molecular approaches including transcriptomics and proteomics will further complement these assessment methods. Assessing fibrosis, a type of -osis or state of the biologic system, can eventually help provide an -omic measure that can be correlated with other -omic measures (e.g., genomics/transcriptomics, proteomics, metabolomics, etc.). Going from the assessment of the -osis can eventually lead to better assessment of -omics. In this sense, fibrosis assessment can act as a biomarker. Ultimately, it is hoped that better assessment will facilitate the development of methods to ameliorate fibrosis and improve patient outcomes (Friedman et al. 2013).
Summary Points
-
This chapter focuses on renal fibrosis, which is the pathologic accumulation of ECM proteins following tissue injury.
-
IF is accompanied by TA, and these are collectively referred to as interstitial fibrosis and tubular atrophy (IFTA).
-
IFTA is shown to have prognostic value in chronic kidney diseases.
-
Renal biopsy can be used to diagnose and quantitate IFTA and further prognosticate renal function.
-
A variety of staining techniques are useful in assessing the severity of IF, including trichrome, picrosirius red, and collagen III immunohistochemistry.
-
Molecular methods such as transcriptional profiling and proteomics can provide surrogate measures of IFTA.
Abbreviations
- BMP:
-
Bone morphogenic protein
- CCR-2:
-
C-C chemokine receptor type 2
- CKD:
-
Chronic kidney disease
- DC:
-
Dendritic cell
- ECM:
-
Extracellular matrix
- EMR-1:
-
EGF-like module-containing mucin-like hormone receptor-like 1
- EMT:
-
Epithelial-mesenchymal transition
- EndoMT:
-
Endothelial-mesenchymal transition
- eNOS:
-
Endothelial nitric oxide synthase
- ERKs:
-
Extracellular-signal-regulated kinases
- FGF-2:
-
Fibroblast growth factor 2
- FSP-1:
-
Fibroblast-specific protein 1
- GFR:
-
Glomerular filtration rate
- GSK:
-
Glycogen synthase kinase
- HGF:
-
Hepatocyte growth factor
- HIF:
-
Hypoxia-induced factor
- IF:
-
Interstitial fibrosis
- IFTA:
-
Interstitial fibrosis/tubular atrophy
- IHC:
-
Immunohistochemical
- ILK:
-
Integrin-linked kinase
- JAK/STAT:
-
Janus kinase/signal transducer and activator of transcription
- LTBP:
-
Latent TGF-β binding protein
- miRNA:
-
MicroRNA
- MMP:
-
Matrix metalloproteinase
- NF-κB:
-
Nuclear factor κB
- PAPMS:
-
Pathogen-associated molecular patterns
- PDGF:
-
Platelet-derived growth factor
- PKC:
-
Protein kinase C
- SMA:
-
Smooth muscle actin
- TA:
-
Tubular atrophy
- TEC:
-
Tubular epithelial cell
- TGF-β:
-
Transforming growth factor-beta
- TIMP:
-
Tissue inhibitors of metalloproteinases
- TLR:
-
Toll-like receptor
- tPA:
-
Tissue plasminogen activator
- TSP-1:
-
Thrombospondin-1
- tTG:
-
Tissue transglutaminase
- USAG-1:
-
Uterine sensitization-associated gene 1 (also known as sclerostin domain-containing protein 1)
- VEGF:
-
Vascular endothelial growth factor
References
Abrass CK, Hansen KM, Patton BL. Laminin alpha4-null mutant mice develop chronic kidney disease with persistent overexpression of platelet-derived growth factor. Am J Pathol. 2010;176(2):839–49.
Agarwal SK, Sethi S, Dinda AK. Basics of kidney biopsy: a nephrologist’s perspective. Indian J Nephrol. 2013;23(4):243–52.
Anders HJ, Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011;80(9):915–25.
Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol. 2010;6(11):643–56.
Bottinger EP. TGF-beta in renal injury and disease. Semin Nephrol. 2007;27(3):309–20.
Bunnag S, Einecke G, Reeve J, Jhangri GS, Mueller TF, Sis B, Hidalgo LG, Mengel M, Kayser D, Kaplan B, Halloran PF. Molecular correlates of renal function in kidney transplant biopsies. J Am Soc Nephrol. 2009;20(5):1149–60.
Choi BS, Shin MJ, Shin SJ, Kim YS, Choi YJ, Kim YS, Moon IS, Kim SY, Koh YB, Bang BK, Yang CW. Clinical significance of an early protocol biopsy in living-donor renal transplantation: ten-year experience at a single center. Am J Transplant. 2005;5(6):1354–60.
Choi HY, Lee HG, Kim BS, Ahn SH, Jung A, Lee M, Lee JE, Kim HJ, Ha SK, Park HC. Mesenchymal stem cell-derived microparticles ameliorate peritubular capillary rarefaction via inhibition of endothelial-mesenchymal transition and decrease tubulointerstitial fibrosis in unilateral ureteral obstruction. Stem Cell Res Ther. 2015;6:18.
Chung AC, Lan HY. MicroRNAs in renal fibrosis. Front Physiol. 2015;6:50.
Doello K. A new pentachrome method for the simultaneous staining of collagen and sulfated mucopolysaccharides. Yale J Biol Med. 2014;87(3):341–7.
Eardley KS, Kubal C, Zehnder D, Quinkler M, Lepenies J, Savage CO, Howie AJ, Kaur K, Cooper MS, Adu D, Cockwell P. The role of capillary density, macrophage infiltration and interstitial scarring in the pathogenesis of human chronic kidney disease. Kidney Int. 2008;74(4):495–504.
Eddy AA. Molecular insights into renal interstitial fibrosis. J Am Soc Nephrol. 1996;7(12):2495–508.
Farris AB, Alpers CE. What is the best way to measure renal fibrosis?: A pathologist’s perspective. Kidney Int Suppl. 2014;4(1):9–15.
Farris AB, Colvin RB. Renal interstitial fibrosis: mechanisms and evaluation. Curr Opin Nephrol Hypertens. 2012;21(3):289–300.
Farris AB, Adams CD, Brousaides N, Della Pelle PA, Collins AB, Moradi E, Smith RN, Grimm PC, Colvin RB. Morphometric and visual evaluation of fibrosis in renal biopsies. J Am Soc Nephrol. 2010;22(1):176-86.
Farris AB, Adams CD, Brousaides N, Della Pelle PA, Collins AB, Moradi E, Smith RN, Grimm PC, Colvin RB. Morphometric and visual evaluation of fibrosis in renal biopsies. J Am Soc Nephrol. 2011;22(1):176–86.
Farris AB, Lawson D, Cohen C, Rosen S. Medullary injury in the human renal biopsy: fibrosis assessment. Lab Investig. 2013;93(Supplement 1):387A–8.
Farris AB, Chan S, Climenhaga J, Adam B, Bellamy CO, Seron D, Colvin RB, Reeve J, Mengel M. Banff fibrosis study: multicenter visual assessment and computerized analysis of interstitial fibrosis in kidney biopsies. Am J Transplant. 2014;14(4):897–907.
Friedman SL, Sheppard D, Duffield JS, Violette S. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med. 2013;5(167), 167sr161.
Furness PN, Taub N, Assmann KJ, Banfi G, Cosyns JP, Dorman AM, Hill CM, Kapper SK, Waldherr R, Laurinavicius A, Marcussen N, Martins AP, Nogueira M, Regele H, Seron D, Carrera M, Sund S, Taskinen EI, Paavonen T, Tihomirova T, Rosenthal R. International variation in histologic grading is large, and persistent feedback does not improve reproducibility. Am J Surg Pathol. 2003;27(6):805–10.
Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris 3rd AB, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M, Banff meeting report writing, committee. Banff 2013 meeting report: inclusion of C4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14(2):272–83.
Habuka M, Fagerberg L, Hallstrom BM, Kampf C, Edlund K, Sivertsson A, Yamamoto T, Ponten F, Uhlen M, Odeberg J. The kidney transcriptome and proteome defined by transcriptomics and antibody-based profiling. PLoS One. 2014;9(12), e116125.
Higgins DF, Kimura K, Iwano M, Haase VH. Hypoxia-inducible factor signaling in the development of tissue fibrosis. Cell Cycle. 2008;7(9):1128–32.
Howie AJ, Brewer DB, Howell D, Jones AP. Physical basis of colors seen in Congo red-stained amyloid in polarized light. Lab Invest. 2008;88(3):232–42.
Huang L, Haylor JL, Hau Z, Jones RA, Vickers ME, Wagner B, Griffin M, Saint RE, Coutts IG, El Nahas AM, Johnson TS. Transglutaminase inhibition ameliorates experimental diabetic nephropathy. Kidney Int. 2009;76(4):383–94.
Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176(1):85–97.
Khan F, Sar A, Gonul I, Benediktsson H, Doulla J, Yilmaz S, Berka N. Graft inflammation and histologic indicators of kidney chronic allograft failure: low-expressing interleukin-10 genotypes cannot be ignored. Transplantation. 2010;90(6):630–8.
Klein J, Kavvadas P, Prakoura N, Karagianni F, Schanstra JP, Bascands JL, Charonis A. Renal fibrosis: insight from proteomics in animal models and human disease. Proteomics. 2011;11(4):805–15.
Konvalinka A, Scholey JW, Diamandis EP. The quest for renal disease proteomic signatures: where should we look? Clin Proteom. 2010;6:45–51.
Kriz W, Kaissling B, Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J Clin Invest. 2011;121(2):468–74.
Kruger T, Benke D, Eitner F, Lang A, Wirtz M, Hamilton-Williams EE, Engel D, Giese B, Muller-Newen G, Floege J, Kurts C. Identification and functional characterization of dendritic cells in the healthy murine kidney and in experimental glomerulonephritis. J Am Soc Nephrol. 2004;15(3):613–21.
Lattouf R, Younes R, Lutomski D, Naaman N, Godeau G, Senni K, Changotade S. Picrosirius red staining: a useful tool to appraise collagen networks in normal and pathological tissues. J Histochem Cytochem. 2014;62(10):751–8.
LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19(8):1047–53.
Lian YG, Zhou QG, Zhang YJ, Zheng FL. VEGF ameliorates tubulointerstitial fibrosis in unilateral ureteral obstruction mice via inhibition of epithelial-mesenchymal transition. Acta Pharmacol Sin. 2011;32:1513–21.
Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173(6):1617–27.
Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69(2):213–7.
Liu L, Kou P, Zeng Q, Pei G, Li Y, Liang H, Xu G, Chen S. CD4+ T Lymphocytes, especially Th2 cells, contribute to the progress of renal fibrosis. Am J Nephrol. 2012;36(4):386–96.
Liu M, Liu YZ, Feng Y, Xu YF, Che JP, Wang GC, Zheng JH. Novel evidence demonstrates that epithelial-mesenchymal transition contributes to nephrolithiasis-induced renal fibrosis. J Surg Res. 2013;182(1):146–52.
Mack M, Rosenkranz AR. Basophils and mast cells in renal injury. Kidney Int. 2009;76(11):1142–7.
Maluf DG, Mas VR, Archer KJ, Yanek K, Gibney EM, King AL, Cotterell A, Fisher RA, Posner MP. Molecular pathways involved in loss of kidney graft function with tubular atrophy and interstitial fibrosis. Mol Med. 2008;14(5–6):276–85.
Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol. 2003;14(5):1358–73.
Meas-Yedid V, Servais A, Noel LH, Panterne C, Landais P, Herve N, Brousse N, Kreis H, Legendre C, Thervet E, Olivo-Marin JC, Morelon E. New computerized color image analysis for the quantification of interstitial fibrosis in renal transplantation. Transplantation. 2011;92(8):890–9.
Mezzano S, Aros C, Droguett A, Burgos ME, Ardiles L, Flores C, Schneider H, Ruiz-Ortega M, Egido J. NF-kappaB activation and overexpression of regulated genes in human diabetic nephropathy. Nephrol Dial Transplant. 2004;19(10):2505–12.
Moreso F, Lopez M, Vallejos A, Giordani C, Riera L, Fulladosa X, Hueso M, Alsina J, Grinyo JM, Seron D. Serial protocol biopsies to quantify the progression of chronic transplant nephropathy in stable renal allografts. Am J Transplant. 2001;1(1):82–8.
Naito T, Ma LJ, Yang H, Zuo Y, Tang Y, Han JY, Kon V, Fogo AB. Angiotensin type 2 receptor actions contribute to angiotensin type 1 receptor blocker effects on kidney fibrosis. Am J Physiol Renal Physiol. 2010;298(3):F683–91.
Niedermeier M, Reich B, Rodriguez Gomez M, Denzel A, Schmidbauer K, Gobel N, Talke Y, Schweda F, Mack M. CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc Natl Acad Sci U S A. 2009;106(42):17892–7.
Paust HJ, Ostmann A, Erhardt A, Turner JE, Velden J, Mittrucker HW, Sparwasser T, Panzer U, Tiegs G. Regulatory T cells control the Th1 immune response in murine crescentic glomerulonephritis. Kidney Int. 2011;80(2):154–64.
Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4(10), e7475.
Prunotto M, Ghiggeri G, Bruschi M, Gabbiani G, Lescuyer P, Hocher B, Chaykovska L, Berrera M, Moll S. Renal fibrosis and proteomics: current knowledge and still key open questions for proteomic investigation. J Proteomics. 2011;74(10):1855–70.
Reich B, Schmidbauer K, Rodriguez Gomez M, Johannes Hermann F, Gobel N, Bruhl H, Ketelsen I, Talke Y, Mack M. Fibrocytes develop outside the kidney but contribute to renal fibrosis in a mouse model. Kidney Int. 2013;84(1):78–89.
Reinders ME, Bank JR, Dreyer GJ, Roelofs H, Heidt S, Roelen DL, Al Huurman V, Lindeman J, van Kooten C, Claas FH, Fibbe WE, Rabelink TJ, de Fijter JW. Autologous bone marrow derived mesenchymal stromal cell therapy in combination with everolimus to preserve renal structure and function in renal transplant recipients. J Transl Med. 2014;12:331.
Rogers NM, Ferenbach DA, Isenberg JS, Thomson AW, Hughes J. Dendritic cells and macrophages in the kidney: a spectrum of good and evil. Nat Rev Nephrol. 2014;10(11):625–43.
Rybinski B, Franco-Barraza J, Cukierman E. The wound healing, chronic fibrosis, and cancer progression triad. Physiol Genomics. 2014;46(7):223–44.
Satoh M, Kashihara N, Yamasaki Y, Maruyama K, Okamoto K, Maeshima Y, Sugiyama H, Sugaya T, Murakami K, Makino H. Renal interstitial fibrosis is reduced in angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol. 2001;12(2):317–25.
Schrimpf C, Xin C, Campanholle G, Gill SE, Stallcup W, Lin SL, Davis GE, Gharib SA, Humphreys BD, Duffield JS. Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J Am Soc Nephrol. 2012;23(5):868–83.
Scian MJ, Maluf DG, Archer KJ, Suh JL, Massey D, Fassnacht RC, Whitehill B, Sharma A, King A, Gehr T, Cotterell A, Posner MP, Mas V. Gene expression changes are associated with loss of kidney graft function and interstitial fibrosis and tubular atrophy: diagnosis versus prediction. Transplantation. 2011;91(6):657–65.
Semedo P, Donizetti-Oliveira C, Burgos-Silva M, Cenedeze MA, Avancini Costa Malheiros DM, Pacheco-Silva A, Camara NO. Bone marrow mononuclear cells attenuate fibrosis development after severe acute kidney injury. Lab Invest J Tech Methods Pathol. 2010;90(5):685–95.
Sethi S, Vrana JA, Theis JD, Dogan A. Mass spectrometry based proteomics in the diagnosis of kidney disease. Curr Opin Nephrol Hypertens. 2013;22(3):273–80.
Snelgrove SL, Kausman JY, Lo C, Ooi JD, Coates PT, Hickey MJ, Holdsworth SR, Kurts C, Engel DR, Kitching AR. Renal dendritic cells adopt a pro-inflammatory phenotype in obstructive uropathy to activate T cells, but do not directly contribute to fibrosis. Am J Pathol. 2011;180(1):91–103
Sorensen I, Susnik N, Inhester T, Degen JL, Melk A, Haller H, Schmitt R. Fibrinogen, acting as a mitogen for tubulointerstitial fibroblasts, promotes renal fibrosis. Kidney Int. 2011;80(10):1035–44.
Street JM, Souza AC, Alvarez-Prats A, Horino T, Hu X, Yuen PS, Star RA. Automated quantification of renal fibrosis with Sirius Red and polarization contrast microscopy. Physiol Rep. 2014;2(7):e12088:1–9.
Sun S, Ning X, Zhai Y, Du R, Lu Y, He L, Li R, Wu W, Sun W, Wang H. Egr-1 mediates chronic hypoxia-induced renal interstitial fibrosis via the PKC/ERK pathway. Am J Nephrol. 2014;39(5):436–48.
Sund S, Grimm P, Reisaeter AV, Hovig T. Computerized image analysis vs semiquantitative scoring in evaluation of kidney allograft fibrosis and prognosis. Nephrol Dial Transplant. 2004;19(11):2838–45.
Tapmeier TT, Fearn A, Brown K, Chowdhury P, Sacks SH, Sheerin NS, Wong W. Pivotal role of CD4+ T cells in renal fibrosis following ureteric obstruction. Kidney Int. 2010;78(4):351–62.
Vilayur E, Harris DC. Emerging therapies for chronic kidney disease: what is their role? Nat Rev Nephrol. 2009;5(7):375–83.
Walker PD, Cavallo T, Bonsib SM, Ad Hoc Committee on Renal Biopsy Guidelines of the Renal Pathology, Society. Practice guidelines for the renal biopsy. Mod Pathol. 2004;17(12):1555–63.
Wang X, Zhou Y, Tan R, Xiong M, He W, Fang L, Wen P, Jiang L, Yang J. Mice lacking the matrix metalloproteinase-9 gene reduce renal interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol. 2010;299(5):F973–82.
Wang Y, Wang B, Du F, Su X, Sun G, Zhou G, Bian X, Liu N. Epigallocatechin-3-gallate attenuates unilateral ureteral obstruction-induced renal interstitial fibrosis in mice. J Histochem Cytochem. 2015;63(4):270–9.
Wilkinson L, Gilbert T, Sipos A, Toma I, Pennisi DJ, Peti-Peterdi J, Little MH. Loss of renal microvascular integrity in postnatal Crim1 hypomorphic transgenic mice. Kidney Int. 2009;76(11):1161–71.
Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D’Agati V, D’Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76(5):546–56.
Xia Y, Entman ML, Wang Y. CCR2 regulates the uptake of bone marrow-derived fibroblasts in renal fibrosis. PLoS One. 2013;8(10), e77493.
Yang J, Shultz RW, Mars WM, Wegner RE, Li Y, Dai C, Nejak K, Liu Y. Disruption of tissue-type plasminogen activator gene in mice reduces renal interstitial fibrosis in obstructive nephropathy. J Clin Invest. 2002;110(10):1525–38.
Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16(5):535–43. 531p following 143.
Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol JASN. 2010;21(11):1819–34.
Zununi Vahed S, Samadi N, Ardalan M. Diagnosis of interstitial fibrosis and tubular atrophy in kidney allograft: implementation of microRNAs. Iran J Kidney Dis. 2014;8(1):4–12.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Dordrecht
About this entry
Cite this entry
Nezami, B.G., Farris, A.B. (2016). Assessing Fibrosis in Kidney Biopsies. In: Patel, V., Preedy, V. (eds) Biomarkers in Kidney Disease. Biomarkers in Disease: Methods, Discoveries and Applications. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7699-9_51
Download citation
DOI: https://doi.org/10.1007/978-94-007-7699-9_51
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7698-2
Online ISBN: 978-94-007-7699-9
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences