Abstract
Organotins are compounds with one or more carbon-tin bonds. Addition of an organo-moiety to tin increases its volatility, its lipid solubility and adsorptivrty, and its toxicity to many organisms. Tin can have a valence of 2+ or 4+ but Sn4+ predominates in the environment. Of the tin mined each year, about 5% is used for production of organotins, yielding some 30,000 tons of organotins (1), and tin may have more of its organometallic derivatives in use than any other element (1,2).
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
1.1 Uses and Importance of Organotins
Organotins are compounds with one or more carbon-tin bonds. Addition of an organo-moiety to tin increases its volatility, its lipid solubility and adsorptivrty, and its toxicity to many organisms. Tm can have a valence of 2+ or 4+ but Sn4+ predominates in the environment. Of the tin mined each year, about 5% is used for production of organotins, yielding some 30,000 tons of organotins (1), and tin may have more of its organometallic derivatives in use than any other element (1,2).
Organotins are widely used in industry and in agriculture. The largest use is as stabrhzers for polyvmyl chlorides followed by their uses as antibiologrcal agents and as chemical catalysts (2,3). As antibiological agents, tri-substituted and di-substituted organotins are the most toxic, and among tri-substituted compounds propyl-, butyl-, pentyl-, phenyl-, and cyclohexyltms are the most toxic (2,4). The anion (X) associated with a tri-substituted organotin (R3SnX) has little effect on toxicity.
1.2 Toxicity of Organotins
Tributyltin (TBT) is a very effective component of antifouling paints applied to surfaces submerged in natural waters. Paints with TBT as their active agent are markedly more effective than paints with the alternative agent, copper (5). TBT leaches out of such paints and, unfortunately, it is toxic to nontarget organisms. Particular attention has been given to the dogwhelk, Nucella Zapihs, which is subject to a TBT-induced condition known as imposex, wherein female organisms grow male sex organs and the population cannot reproduce (6,7). Imposex can occur at concentrations as low as 1 ng TBT-Sri/L (1 part per trillion, 8.4 PM) (8,9) Molluscs exposed to TBT may be unusually sensitive because they have low activities of mixed function oxidases and cytochrome P-450 that leads to TBT accumulation (10). Accumulated TBT leads to an increase in testosterone (11)
TBT causes morphological abeviations in mussels and oysters (12), and a variety of other micro- and macroorganisms are sensitive to it (2). Debutylation in the sequence TBT → DBT (dibutyltion) → MBT (monobutyltin) → Sn (inorganic) detoxifies TBT for most macroorgamsms but not for some microorganisms (4,14-16). Moreover, TBT and its degradation products can act as negative chemotactic agents for aquatic bacteria at concentrations over four orders of magnitude lower than the concentrations that Inhibit growth of the orgamsms (17) Thus, TBT and its degradation products may have effects on microbial populations at concentrations far lower than concentrations that inhibit growth. MBT is as toxic as TBT to some microorgamsms, including bacteria (18), yeasts (14), and the replication of bacteriophage T4 (19).
1.3 TBT in the Aquatic Environment
Organic and inorganic tin compounds can be concentrated up to lO,OOO-fold in the surface microlayer and up to 4000-fold in oily sediments (1,20,21). They are also found in water and sludge in sewage treatment plants (22). Most of the pollutant tin in aquatrc systems is attached to suspended particles (23,24) but it may also be associated with dissolved organic matter (25,26). Concentrations are about three orders of magnitude higher in sediments than in the water column (27-29). Since TBT leaches from antifoulmg paints, concentrations of it and its degradation products, DBT and MBT, are higher in harbors and marinas than in open water (1,2,27,30-32). Recently deposited sediments contain about ten times as much tin as sediments deposited before the Industrial Revolution (33). Sediments are a probable source of organotins for benthic deposit feeders (34) and it is to be expected that butyltins will interact strongly with benthic organisms.
TBT can be degraded in the water column with a half-life of days (35) and photolysis may contribute to the degradation (25). Biological and chemical degradation in sediments is slower, with a half-life of months (24), years (36), or much longer (37).
1.4 Regulation of TBT
Because of its effects on nontarget organisms, the use of TBT in antlfoulmg paints has been regulated in a number of countries. In 1982 in France, followed by the United Kingdom, Ireland, Germany, and Switzerland Ireland regulated all organotms, although other countries regulated only TBT. The initial British regulation restricted the amount of TBT in antifouling paints, but did not restrict their use. The regulation did not have the desired effect, and in 1987 the use of TBT-paints was banned on small boats and mariculture equipment (38). In 1988 the use of TBT was regulated in the United States. The regulations are similar in all countries: TBT-containing paints may not be used on boats shorter than 25 in. Aluminum-hulled boats are exempt because copper-based paints can be involved in hull electrolysis (39).
Decreased use of paints with TBT has reduced the amounts of TBT in the environment (39-42) Levels in sediments have not decreased as rapidly as those in water (43) and levels exceed acceptable levels at many sites (44), although effects on shellfish populations have decreased (42,43,45,46).
Many countries have not regulated the use of TBT, and paints have been developed that contain trlphenyltin (TPhT) as the active agent, which is not covered by the regulations in most countries. It is necessary to measure TBT, TPhT, and their degradation products in the environment now and it will be necessary to do so in the future.
1.5 Approaches to Quantifying Tin Compounds
Although some environmental issues will require measurement of only total tin in the environment, the differences in toxicity among the several organotins will generally dictate measurement of mdivldual organotins. Thus, it is necessary to separate the several tin compounds and to quantify them individually. The tin compounds must be removed from particulates to which they may adhere. The individual compounds are easier to separate as derivatives, so they are converted to hydrides or to Grignard products, separated and concentrated, and then quantified. In most methods, quantification is based on the amount of tin in each individual derivative. Thus, separation is based on the organomoiety and quantification is based on the tin moiety.
There are a number of methods available. The one presented here has been used in our laboratory, particularly for sediments (47- 49).
2 Materials
-
1.
The internal standard is trlethyltm bromide obtained from Aldrich Chemical Company (Milwaukee, WI)
-
2.
The hydride reaction vessel is as described by Donard et al (50) and can be handcrafted by Anderson Glass, Inc. (Atzwilliam, NH)
-
3.
NaBH4, is from Aldrich and is 6%. It is filtered through a 0.4μm pore size glass filter and allowed to stand overnight, after which helium is bubbled through the solution to reduce contamination by inorganic Sn
-
4.
A chromatographlc trap consists of a 45cm length of 6-mm ID Teflon tubing Inserted inside a glass U-tube wrapped with 26-gage nlchrome wire attached to a vanable transformer (Variac) The U-tube is shaped to fit inside a 285-mL vacuum Dewar flask containing liquid nitrogen, which cools the trap to -170°C The Teflon tube is inserted in the glass tube in order to shield it from localized heat of the nichrome wire. The column packmg matenal is Chromosorb G 45/60 mesh AW-DCMS, coated with either 3% SP-2100 (Supelco Inc., Bellefonte, PA) or 3% OV-101 (Altech, Deerfield, IL).
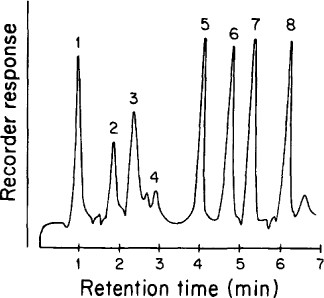
Fig. 1. Quartz furnace. From ref. 50, by permission
-
5.
The packing material for the chromatographic trap is Chromosorb G 45/60 mesh AW-DCMS, coated with either 3% SP-2100 (Supelco Inc., Bellefonte, PA) or 3% OV-101 (Altech, Deerfield, IL)
-
6.
The quartz furnace (Fig 1.) is as described by Donard et al (50). It was obtained from Anderson Glass Furnaces are wrapped with ceramic tape (Wale Apparatus Co., Hellertown, PA) instead of the asbestos tape described by Donard et al. (50)
-
7.
Authentic methyl- and butylin compounds are from Alfa Products (Danvers, MA)
3 Method
3.1 Sampling
Sediment samples are collected with an Ekman dredge, or by hand with a glass beaker (see Note 1). Material from the top 2 cm that had not been in contact with the metal dredge is scraped into acid-cleaned glass jars with the aid of a small glass beaker or subsamples are taken with a polypropylene corer (the outer portion of a syringe). Samples are stored on me during transport and, if not analyzed immedrately, they are then frozen and stored at -4°C under air in the dark until they are extracted. If cores are used, they can be extruded and sampled, taking care to take material which has not been in contact with a metal surface
3.2 Removal of Tin Compounds from Particulates
-
1.
Place approx 10 g of wet sediment in a 250-mL round-bottomed flask Use a duphcate sample to determme dry weight after drymg it overnight at 60°C
-
2.
Add 100 p.L of a solution containing 10 μg/mL of Sn as trtethyltin bromide to the flask as an internal standard followed by 0.5 mL of concentrated reagent grade HCL
-
3.
Swirl the flask for 30 s and add 20 mL of HPLC grade methanol.
-
4.
Place a Teflon-covered strringg bar in the flask and reflux the mixture for 1 h at 80°C using a heating mantle and a magnetic stirrer
-
5.
Allow the sample to cool to room temperature and transfer the slurry to a 50-mL polycarbonate centrifuge tube
-
6.
Centrifuge the sample at 270g for 10 mm and transfer the supernatant phase to a glass vial usmg a Pasteur pipet
-
7.
Seal the vial with a Teflon septum cap.
-
8.
Transfer a 1.5-mL ahquot of this fluid to a hydride reaction vessel containing 100 mL of acidified water (pH 2 8) and a Teflon-coated stirring bar
3.3 Hydridization and Concentration
The procedure involves reacting an aqueous sample containing organotin(s) with NaBH4 to convert the tm compounds to their volatile tin hydndes, whtch are then swept onto a cold chromatographic trap. The hydride generation apparatus was modified from the apparatus described by Donard et al. (50). For water samples, see Note 2.
-
1.
Begin stirring and bubble helium through the sample for l-2 mm
-
2.
Inject 2 5 mL of 6% NaBH4 through a septum-stoppered port.
-
3.
After 2 mm, Inject another 2 5 mL into the reaction vessel and allow it to react for an additional 4 min. The volatile hydrides formed are swept by the stream of helium into a chromatographtc trap.
3.4 Separation and Quantification
-
1.
Divert helium flow from the reaction vessel via a four-way Hamilton valve and remove the trap from the liquid nitrogen.
-
2.
Elute individual hydrides from the column by first allowing the trap to warm to room temperature for 3 5 min to allow methyltin hydrides to elute; then turn the Varrac up to 25 V for 1 .5 mm and then to 35 V for 2 min to elute triethyltin and butyltin hydrides The final temperature of the column is 230°C.
-
3.
As individual hydrides are eluted from the column, they are swept by the helium into a quartz furnace ahgned in the beam of an atomic absorption spectrophotometer (AAS) (see Chapter 15).
-
4.
The AAS is an IL 551 with a hollow cathode tin lamp (Fisher Sctentifrc). Set the wavelength at 224.5 nm with a bandwrdth of 0.5 nm
-
5.
Record peak areas with a Hewlett Packard 3390A integrator Start the integrator when the chromatographtc trap is removed from the hqurd nitrogen.
Quanttfrcation of butyltins involves the method of additions, with MBT, DBT, and TBT spokes added to the reaction vessel.
Separation of authentic tin compounds by hydndization, purge and trap, and atomic absorption spectrometry Numbered peaks Indicate 1 SnH4, 2. CH3SnH3, 3 (CH3)3 SnH2, 4 unidentified peak associated with methanol used as solvent, 5 (C4H9) SnH3, 6 (C2H5)3 SnH, 7 (C4H9) 2SnH2, 8 (C4H9) 3SnH Reproduced from ref 47, with permission
3.5 Analysis of a Typical Sample
Authentic methyl and butyltin compounds placed in the hydride reaction vessel are readily separated and quantified (Fig. 2) (see Note 3), with the exception of trimethyltin, which is masked by a contaminant present in HPLC-grade methanol obtained from two suppliers. Inorganic tin is a contaminant in NaBH4. Results from analysis of a typical sample are shown in Fig. 3. Recoveries of triethyltin, which is added to sediment samples prior to their extraction with acldified methanol, range from 64 to 93%. In comparison, Mathias et al. (51) obtained recoveries of 79-140% when tributyltin was added to sediments that were then extracted with acidified methanol as in the present work, separated by gas chromatography and quantified by flame photometry. Hattori et al. (52) reported recoveries of 68-93% of di- and trialkyltms from sediments using acidlfied methanol, extraction into benzene, clean-up on slhca gel columns, and borohydride derivatization followed by gas chromatography with detection by electron capture. Maguire et al. (53) obtained recoveries of 55 ± 26 to 180 ± 100% for butyltins after benzene-tropolone extraction of acidified sediments, formation of pentyl-derivatives, silica gel clean-up, and analysis by GC usmg a flame photometric detector
When 10 g wet weight of sediment was analyzed, the limits of detection (LOD) values were approx 0.4 ng of organotm/g of sediment, based on LODs of approx 1 ng of each compound/100 mL of fluid in the hydride reaction vessel (see Note 4). Reproducibilrty was good among replicate samples, yielding values ±0-10.6% of the average for TBT, ±1.7-9.3% for DBT, and ±0-4.5% for MBT (48).

Tin compounds detected in a sediment sample from Marma Bay, Boston Harbor, MA Numbered peaks indicate. 1 Sn4H, la Unidentified compound, 5 (C4H9) SnH3, 6. (C2H5)3 SnH4 used as a recovery surrogate that was added to the sedtment before analysis, 7. (C4H9)2, SnH2, 8. (C4H9)3 SnH. Reproduced from ref. 47, with permtssron
4 Notes
-
1.
Care should be taken to avoid metal contamination from the dredge or other sampler. Similarly, polyvinylchlorides leach small amounts of tin Samples should be handled with the knowledge that inorganic tin is strongly adsorbed onto glass surfaces but not very much onto plastics, and the reverse is true for organotins All glassware IS first cleaned, rinsed three times with water that has been deromzed, drstrlled and redistilled in a quartz still, and then either soaked overnight in warm 10% hydrochloric or nitric or rinsed with 10% acid before use, followed by an additional three rinses in quartz-drstrlled water
-
2.
Water samples are analyzed by acidifying them to pH 2 8, placing 100 mL in the hydride reaction vessel, and proceeding as for methanol extracts of sediments.
-
3.
Other organotins could undoubtedly be separated and quantified by adjusting the temperature regimen used in elutmg hydride derivatives from the chromatographrc column.
-
4.
The sensitivity could be Increased by extracting a larger quantity of sediment and increasing the volume of the methanol extract placed in the hydrodrzatron vessel
References
Magmre, R J (1987) Environmental aspects of trtbutyltin Appl Organomet Chem 1, 475–498
Thompson, J A.J, Sheffer, M G., Pierce, R. C., Chau, Y K., Cooney, J. J., and Magune, R J (1985) Organotin Compounds in the Aquatic Environment National Research Council Canada, Ottawa, 284 pp.
Blunden, S J., Hobbs, L A., and Smith, P J. (1984) The environmental chemistry of organotm compounds in Environmental Chemistry (Bowen, H. J., ed ), Royal Soc. Chem., London, pp. 49–77
Cooney, J J and Wuertz, S (1989) Toxic effects of tin compounds on microorganisms J Ind Microbiol 4 375–402.
Champ, M A and Pugh, W L (1987) Tributyltm antifouling paints introduction and overview Oceans 87 4, 1296–1308.
Gibbs, P E., Bryan, G W., and Pascoe, P L (1992) TBT-induced imposex in the dogwhelk, Nucellus lapcllus geographical uniformity of the response and effects Mar Environ Res 32, 79–87
Spence, S K., Bryan, G W., Gibbs, P E., Masters, D., Morris, L., and Hawkins, S J. (1990) Effects of TBT contamination on Nucella populations Funct Ecol 4, 425–432
Gibbs, P. E and Bryan, G W. (1986) Reproductive failure in populattons of the dogwhelk, Nucella laplllus, caused by imposex induced by tributyltin from anttfouling paints. J Mar. Biol Assoc U.K. 66, 767–777
Oehlmann, J., Stroben, E., and Fiormi, P. (1991) The morphological expression of imposex in Nucella lapillus (Linnaeus) (Gastropoda Muricidae) J. Molluscan Stud., 57, 375–390.
Lee, R. F (1991) Metabolism of trtbutyltin by marine animals and possible linkages to effect. Mar. Environ Res. 32, 29–35
Spooner, N., Gibbs, P E., Bryan, G W., and Goad, L J (1991) The effect of tributyltin upon steroid titres in the female dogwhelk, Nucella lupillus, and the development of imposex. Mar. Envwon. Res. 32, 37–49
Waldock, M. J. and Tham, J E. (1983) Shell thickening in Crassostria gigas’ organotin anttfouling or sediment induced? Mar. Pollut. Bull. 14, 411–415.
Hall, L W., Jr and Pinkney, A. E. (1985) Acute and sublethal effects of organotin compounds on aquatic biota an interpretative literature evaluation. CRS Crtt Rev Toxicol 14, 159–209.
Cooney, J J., DeRome, L., Laurence, O., and Gadd, G M. (1989) Effects of organotin and organolead compounds on yeasts J. Ind Microbiol. 4, 279–288.
Uchida, M. (1993) Inhibitory activity of organotin compounds against colony formation of estuarine bacteria Nippon Suzsan Gakkazshi 59, 2037–2042
Cooney, J. J (1995) Organotin compounds and aquatic bacteria. a review Helgolander Meeresuntersuchungen 49, 663–677
Han, G. and Cooney, J J. (1995) Effects of butyltins and inorganic tin on chemo-taxis of aquatic bacteria. J Ind. Microbiol. 14, 293–299.
Boopathy, R. and Daniels, L. (1991) Pattern of organotin inhibition of methanogenic bacteria. Appl Environ Microbiol. 57, 1189–1193
Doolittle, M. M. and Cooney, J. J (1992) Inactivatton of bacteriophage T4 by organic and inorganic tin compounds. J Ind. Microbiol. 10, 221–228.
Cleary, J J. and Stebbing, A R D. (1985) Organotin and total tin in coastal waters of southwest England. Mar Pollut Bull 16, 350–355
Magmre, R J and Tkacz, R K (1987) Concentration of tributyltin in the surface microlayer of natural waters Water Pollut. Res. Can. 22, 227–233.
Donard, O. F X., Quevauviller, P, and Bruchet, A. (1993) Tin and organotin specia-non durmg wastewater and sludge treatment processes Water Res 27, 1085–1089
Byrd, J. T and Andreae, M. O. (1982) Tin and methyltin species in seawater. concentrations and fluxes Science 218, 565–569
Maguire, R J and Tkacz, R. K (1985) Degradation of tri-n-butyltin species in water and sediment from Toronto Harbor. J. Agrc. Food Chem 33, 947–953
Froehch, P. N., Kaul, L, Byrd, J T, Andreae, M O., and Roe, K. K. (1985) Arsenic, barium, germanium, tin, dimethylsulfide and nutrient biogeochemistry in Charlotte Harbor, Florida, a phosphorus-enriched estuary. Estuarine Coastal, Shelf Set 20, 239–264.
Donard, O F X. and Weber, J H. (1985) Behaviour of methyltin compounds under simulated condtions Environ Set. Technol. 10, 901–908
Valkirs, A. O., Seligman, P. F, and Lee, R. F. (1986) Butyltin partitionmg in marine waters and sediments. Oceans 87 4, 1381–1385.
Fent, K. and Hunn, J (1991) Phenyltins in water, sediment and biota of freshwater marinas Environ. Set. Technol. 25, 956–963
Schebek, L. and Andreae, M. O (1991) Methyl-and butyltin compounds in water and sediments of the Rhine River Environ. Sci. Technol. 25, 871–878.
Alzieu, C., Michel, P, SanJuan, J., and Averty, B (1990) Tributyltin levels in French Mediterianean coastal waters. Appl Organomet. Chem 4, 55–61
Cullen, W. R., Eigendorf, G K., Nwata, B. U. and Takatsu, A. (1990) The quantitation of butyltin and cyclohexyltin compounds in the marine environment of British Columbia. Appl Organomet Chem. 4, 581–590.
Wuertz, S., Miller, C. E., Pfister, R. M., and Cooney, J. J. (1991) Tributyltin-resis-tant bacteria from estuarme and freshwater sediments. Appl Environ Microbiol. 57, 2783–2789.
Seidel, S. V., Hodge, V. F., and Goldberg, E D (1980) Tin as an environmental pollutant Thalassta Jugosl 16, 209–223
Langston, W. J. and Burt, G. R. (1991) Bioavailability and effects of sediment-bound TBT in deposit-feeding clams, Scrobularia plana Mar. Envtron. Res. 32, 61–67
Sehgman, P. F, Magmre, R. J., Lee, R F, Hinga, K. R., Valkirs, A O and Stang, P. M. (1996) Persistence and fate of tributyltin in aquatic ecosystems, in Organotins Envtronmental Fates and Effects (Champ, M. and Sebgman, P. F., eds.), Elsevier, London, in press
deMora, S. J., King, N. G., and Miller, M. C. (1989) Tributyltin and total tin in sediment: profiles and the apparent rate of TBT degradation. Environ. Technol. Lett. 10, 901–908.
Adelman, D., Hinga, K R., and Prison, M. E Q (1990) Biogeochemistry of butyltins in an enclosed marine ecosystem Environ. Sci. Technol. 24, 1027–1032.
Vosser, J L (1987) Antlfouling paints new release Dept of Environment, London
Huggett, R J., Unger, M A., Sehgman, P. F, and Valkris, A O. (1992) The marine btocide tnbutyltin. Environ. Sci. Technol. 26, 232–237
Alzreu, C (1991) Environmental problems caused by TBT in France assesment, regulatrons, prospects. Mar Environ. Res 32, 7–17
Cleary, J J. (1991) Organotin in the marine surface microlayer and subsurface waters of south-west England relatron to toxtcrty thresholds and the U.K. envnonmental quality standard Mar Environ. Res. 32, 213–222
Minchin, D., Oehlmann, J., Duggan, C. B., Stroben, E., and Keatinge, M. (1995) Marine TBT antifouling contamination in Ireland, following legtslatron in 1987 Mar Pollut. Bull 30, 633–639
Wane, M. E., Waldock, M. J., Tham, J E., Smith, D J., and Milton, S M (1991) Reductions in TBT concentrations n-r UK estuaries following legrslatton in 1986 and 1987 Mar Environ Res 32, 89–111
Alzieu, C., Michel, P., Tolosa, I, Bacct, E., Mee, L D., and Readman, J W (1991) Organotin compounds in the Mediterianean a continuing cause for concern. Mar Environ Res 32, 261–270
Alzieu, C. (1986) TBT detrimental effects on oyster culture in France— evolution since antrfouling paint regulation Oceans 86 4, 1130–1134
Bailey, S. K and Davies, I M (1991) Continuing Impact of TBT, previously used in martculture, on dogwhelk (Nucella lapillus L) populations in a Scottish sea loch Mar Environ Res. 32, 187–199.
Cooney, J J., Kronick, A. T., Olson, G. J., Blair, W. R, and Brinckman, F E (1988) A modtfied method for quanttfymg methyl and butyltins in estuarme sedrments. Chemosphere 17, 1795–1802
Makkar, N S., Kronick, A T, and Cooney, J J. (1989) B utyltins in sediments from Boston Harbor, USA. Chemosphere l8 2043–2050
Wuertz, S., Miller, M E., Doolittle, M M., Brennan, J F., and Cooney, J J. (1991) Butyltins in estuarme sedrments two years after tributyltin use was restricted Chemosphere 22, 1113–1120
Donard, O. F X., Rapsomanikis, S., and Weber, J H (1986) Speciatron of inorgame tin and alkyltin compounds by atomic absorptron spectrometry using electrothermal quartz furnace after hydride generation Analyt. Chem. 58, 772–777
Matthras, C. L., Bellama, J M., Olson, G. J., and Brinckman, F E (1989) Determination of dr-and tributyltin in sediment and microbial brofrlms using actdified methanol extraction, sodium borohydride denvattzation and gas chromatogra-phy with flame photometric detection Int. J Environ. Analyt. Chem. 35, 61–68
Hattort, Y., Kobayashr, A., Takemoto, S., Takaml, K., Kuge, Y., Sugimae, A., and Nakamoto, M. (1984). Determmatron of trtalkyltin, dralkyltin, and trtphenyltin compounds in envnonmental water and sediments J Chromatog 315, 341–349
Maguire, R J., Tkacz, R J., Chau, Y K., Bengert, G A., and Wong, P T S (1986) Occurence of organotin compounds in water and sedrment in Canada Chemosphere 15, 253–274
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 1997 Humana Press Inc, Totowa, NJ
About this protocol
Cite this protocol
Cooney, J.J. (1997). Quantifying Organic and Inorganic Tin Compounds in Environmental Samples. In: Bioremediation Protocols. Methods in Biotechnology™, vol 2. Humana Press. https://doi.org/10.1385/0-89603-437-2:213
Download citation
DOI: https://doi.org/10.1385/0-89603-437-2:213
Publisher Name: Humana Press
Print ISBN: 978-0-89603-437-2
Online ISBN: 978-1-59259-482-5
eBook Packages: Springer Protocols






