Abstract
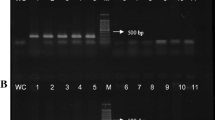
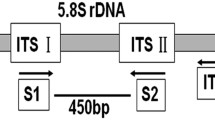
Spread of Verticillium wilt into newly established olive orchards in Andalucía, southern Spain, has caused concern in the olive industry in the region. This spread may result from use of Verticillium dahliae-infected planting material, which can extend distribution of the highly virulent, defoliating (D) pathotype of V. dahliae to new areas. In this study, a molecular diagnostic method for the early in planta detection of D V. dahliae was developed, aimed especially at nursery-produced olive plants. For this purpose, new primers for nested PCR were designed by sequencing a 992-bp RAPD marker of the D pathotype. The use of the specific primers and different nested-PCR protocols allowed the detection of V. dahliae pathotype D DNA in infected root and stem tissues of young olive plants. Detection of the pathogen was effective from the very earliest moments following inoculation of olive plants with a V. dahliae pathotype D conidia suspension as well as in inoculated, though symptomless, plants.
Similar content being viewed by others
References
Akillioğlu M and Tanriserver A (1997) Descripción de los compuestos fenólicos en el olivo y determinación de la composición fenólica en dos diferentes órganos y cultivares. Olivae 68: 28–31
Al-Ahmad MA and Mosli MN (1993) Verticillium wilt of olive in Syria. Bulletin OEPP/EPPO Bulletin 23: 521–529
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W and Lipman DJ (1997) Gapped BLAST and PSIBLAST: a new generation of protein database search programs. Nucleic Acids Research 25: 3389–3402
Bahnweg G, Schulze S, Möller EM, Rosenbrock H, Langebartels C and Sanderman H (1998) DNA isolation from recalcitrant materials such as tree roots, bark, and forest soil for the detection of fungal pathogens by polymerase chain reaction. Analytical Biochemistry 262: 79–82
Banfield WM (1941) Distribution in the sap stream of spores of three fungi that induce vascular wilt diseases of elm. Journal of Agricultural Research 62: 637–681
Bejarano-Alcázar J, Blanco-López MA, Melero-Vara JM and Jiménez-Díaz RM (1996) Etiology, importance, and distribution of Verticillium wilt of cotton in southern Spain. Plant Disease 80: 1233–1238
Blanco-López MA, Jiménez-Díaz RM and Caballero JM (1984) Symptomatology, incidence and distribution of Verticillium wilt of olive trees in Andalucia. Phytopathologia Mediterranea 23: 1–8
Carder JH, Morton A, Tabrett AM and Barbara DJ (1994) Detection and differentiation by PCR of subspecific groups within two Verticillium species causing vascular wilts in herbaceous hosts. In: Schots A, Dewey FM and Oliver R (eds) Modern Assays for Plant Pathogenic Fungi: Identification, Detection and Quantification (pp 91–97) CAB International, Oxford
De Boer SH, Ward LJ, Li X and Chittaranjan S (1995) Attenuation of PCR inhibition in the presence of plant compounds by addition of BLOTTO. Nucleic Acids Research 23: 2567–2568
Emechebe AM, Leaky CLA and Banage WB (1975) Verticillium wilt of cacao in Uganda: incidence and progress of infection in relation to time. East African Agricultural and Forestry Journal 41: 184–186
Heale JB (2000) Diversification and speciation in Verticillium — an overview. In: Tjamos EC, Rowe RC, Heale JB and Fravel DR (eds) Advances in Verticillium Research and Disease Management (pp 1–14) APS Press, St Paul, MN
Heinz R, Lee SW, Saparno A, Nazar RN and Robb J (1998) Cyclical systemic colonization in Verticillium-infected tomato. Physiological and Molecular Plant Pathology 52: 385–396
Hu X, Nazar RN and Robb J (1993) Quantification of Verticillium biomass in wilt disease development. Physiological and Molecular Plant Pathology 42: 23–36
Jiménez-Díaz RM, Tjamos EC and Cirulli M (1998) Verticillium wilt of major tree hosts: olive. In: Hiemstra JA and Harris DC (eds) A Compendium of Verticillium Wilt in Trees Species (pp 13–16) Ponsen & Looijen, Wageningen
López-Escudero J (1999) Evaluación de la resistencia de olivo a las variantes patogénicas de Verticillium dahliae y eficacia de la solarización en el control de la Verticilosis. Ph.D. Thesis, University of Córdoba, Spain
Mercado-Blanco J, Rodríguez-Jurado D, Pérez-Artés E and Jiménez-Díaz RM (2001) Detection of the nondefoliating pathotype of Verticillium dahliae in infected olive plants by nested PCR. Plant Pathology 50: 609–619
Morton A, Carder JH and Barbara DJ (1995) Sequences of the internal transcriber spacers of the ribosomal RNA genes and relationships between isolates of Verticillium albo-atrum and V. dahliae. Plant Pathology 44: 183–190
Moukhamedov R, Hu X, Nazar RN and Robb J (1994) Use of polymerase chain reaction amplified ribosomal intergenic sequences for the diagnosis of Verticillium tricorpus. Phytopathology 84: 256–259
Nazar RN, Hu X, Schmidt J, Culham D and Robb J (1991) Potential use of PCR-mediated ribosomal intergenic sequences in the detection and differentiation of Verticillium wilt pathogens. Physiological and Molecular Plant Pathology 39: 1–11
Pérez-Artés E, García-Pedrajas MD, Bejarano-Alcázar J and Jiménez-Díaz RM (2000) Differentiation of cotton-defoliating and-nondefoliating pathotypes Verticillium dahliae by RAPD and specific PCR analyses. European Journal of Plant Pathology 106: 507–517
Raeder U and Broda P (1985) Rapid preparation of DNA from filamentous fungi. Letters of Applied Microbiology 1: 17–20
Robb J, Hu X, Platt H and Nazar R (1994) PCR assay for the detection and quantification of Verticillium species in potato. In: Schots A, Dewey FM and Oliver R (eds) Modern Detection Assay for Plant Pathogenic Fungi: Identification, Detection and Quantification (pp 83–90) CAB International, Oxford
Rodríguez-Jurado D (1993) Interacciones huésped-parásito en la Verticilosis del olivo (Olea europaea L.) inducida por Verticillium dahliae Kleb. Ph.D. Thesis, University of Córdoba, Spain
Rodríguez-Jurado D, Blanco-López MA, Rapoport HF and Jiménez-Díaz RM (1993) Present status of Verticillium wilt of olive in Andalucia (southern Spain). Bulletin OEPP/EPPO Bulletin 23: 513–516
Rollo F, Salvi R and Torchia P (1990) Highly sensitive and fast detection of Phoma tracheiphila by polymerase chain reaction. Applied and Microbiology Biotechnology 29: 572–576
Sambrook J, Fristsch EF and Maniatis T (1998) Molecular Cloning: A Laboratory Manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Sánchez-Hernández ME, Ruíz-Dávila A, Pérezde Algaba A, Blanco-López MA and Trapero-Casas A (1998) Occurrence and etiology of death of young olives trees in southern Spain. European Journal of Plant Pathology 104: 347–357
Schnathorst WC and Mathre DE (1966) Host range and differentiation of a severe form of Verticillium albo-atrum in cotton. Phytopathology 56: 1155–1161
Schnathorst WC and Sibbett GS (1971) The relation of strains of Verticillium albo-atrum to severity of Verticillium wilt in Gossypium hirsutum and Olea europaea in California. Plant Disease Reporter 9: 780–782
Thanassolopoulos CC (1993) Spread of Verticillium wilt by nursery plants in olive growes in the Chalkidiki area (Greece). Bulletin OEPP/EPPO Bulletin 23: 517–520
Thanassoulopoulos CC, Biris DA and Tjamos EC (1979) Survey of Verticillium wilt of olive trees in Greece. Plant Disease Reporter 63: 936–940
Tjamos EC (1993) Prospects and strategies in controlling Verticillium wilt of olive. Bulletin OEPP/EPPO Bulletin 23: 505–512
Tsukamoto H, Hisada S, Nishibe S and Roux DG (1984) Phenolic glycosides from Olea europaea subspc. Africana. Phytochemistry 23: 2839–2841
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mercado-Blanco, J., Rodríguez-Jurado, D., Pérez-Artés, E. et al. Detection of the Defoliating Pathotype of Verticillium dahliae in Infected Olive Plants by Nested PCR. European Journal of Plant Pathology 108, 1–13 (2002). https://doi.org/10.1023/A:1013994827836
Issue Date:
DOI: https://doi.org/10.1023/A:1013994827836