Abstract
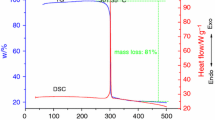
The thermal kinetic performance and storage life of tris(carbohydrazide)manganese(II) perchlorate (GTM), tris(carbohydrazide)nickel(II) perchlorate (GTN), tris(carbohydrazide)zinc(II) perchlorate (GTX), and tris(carbohydrazide)cadmium(II) perchlorate (GTG), as important high-energy and green materials, were carried out by the DSC, (thermogravimetric) TG, and a dynamic pressure measuring thermal analysis (DPTA) method. The thermal behavior, kinetics, thermal safety, and storage life of them were investigated. The results show that there are three mass-loss stages in TG curves, and one endothermic peak and two exothermic peaks in DSC curve for them. The first mass-loss stages are the melting processes, and the thermal decompositions have happened in this stage. The kinetic data were obtained from the DSC and TG curves by integral and differential methods. The most probable kinetic models and kinetic equations were suggested by polynomial fitting the kinetic data. The specific heat capacity was determined with theoretical calculation method, and then self-accelerating decomposition temperature (T SADT), thermal ignition temperature (T TIT), critical temperatures of thermal explosion (T b), and the adiabatic time-to-explosion (t TIAD) are calculated, respectively. The storage lives of 0.01 % conversion rate for GTM, GTN, GTX, and GTG at 25 °C are 4.52a (annual), 9.26a 10.75a, and 7.57a. GTX is the most excellent carbohydrazide perchlorate.


Similar content being viewed by others
References
Chavez DE. The development of environmentally sustainable manufacturing technologies for energetic materials. Green Energ Mater. 2014;235–258.
Olah GA, Squire DR. Chemistry of energetic materials. Waltham: Academic press; 1991.
Furman D, Kosloff R, Dubnikova F, Zybin SV, Goddard WA III, Rom N, et al. Decomposition of condensed phase energetic materials: interplay between uni-and bimolecular mechanisms. J Am Chem Soc. 2014;136(11):4192–200.
Zhang Q, Shreeve JnM. Metal-organic frameworks as high explosives: a new concept for energetic materials. Angew Chem Int Ed. 2014;53(10):2540–2.
Pagoria PF, Lee GS, Mitchell AR, Schmidt RD. A review of energetic materials synthesis. Thermochim Acta. 2002;384(1):187–204.
Sikder A, Sikder N. A review of advanced high performance, insensitive and thermally stable energetic materials emerging for military and space applications. J Hazard Mater. 2004;112(1):1–15.
Badgujar D, Talawar M, Asthana S, Mahulikar P. Advances in science and technology of modern energetic materials: an overview. J Hazard Mater. 2008;151(2):289–305.
Talawar M, Sivabalan R, Mukundan T, Muthurajan H, Sikder A, Gandhe B, et al. Environmentally compatible next generation green energetic materials (GEMs). J Hazard Mater. 2009;161(2):589–607.
Figen AK, Atali PY, Pişkin MB. Thermal properties and kinetics of new-generation posterior bulk fill composite cured light-emitting diodes. J Therm Anal Calorim. 1–12.
Deng J, Wang K, Zhang Y, Yang H. Study on the kinetics and reactivity at the ignition temperature of Jurassic coal in North Shaanxi. J Therm Anal Calorim. 1–7.
Gil A, Barreneche C, Moreno P, Solé C, Inés Fernández A, Cabeza LF. Thermal behaviour of d-mannitol when used as PCM: comparison of results obtained by DSC and in a thermal energy storage unit at pilot plant scale. Appl Energy. 2013;111:1107–13.
López-González D, Fernandez-Lopez M, Valverde J, Sanchez-Silva L. Kinetic analysis and thermal characterization of the microalgae combustion process by thermal analysis coupled to mass spectrometry. Appl Energy. 2014;114:227–37.
Tang Z, Ren Y, Yang L, Zhang T, Qiao X, Zhang J, et al. Researches on thermal decomposition kinetics of composite modified double-base propellants. Chin J Chem. 2011;29(3):411–4.
Wang S, Yang L, Zhang T, Zhang G, Zhang J, Zhou Z. Synthesis, crystal structure, thermal decomposition, and explosive properties of Bi(tza)(3) (n) (tza = tetrazole acetic acid). J Coord Chem. 2011;64(15):2583–91.
Wu B, Wang S, Yang L, Zhang T, Zhang J, Zhou Z, et al. Preparation, crystal structures, thermal decomposition and explosive properties of two novel energetic compounds M (IMI) 4 (N3) 2 (M = CuII and NiII, IMI = Imidazole): the new high-nitrogen materials (N > 46 %). Eur J Inorg Chem. 2011;2011(16):2616–23.
Comesaña R, Gómez M, Álvarez Feijoo M, Eguía P. CFD simulation of a TG–DSC furnace during the indium phase change process. Appl Energy. 2013;102:293–8.
Lazaro A, Peñalosa C, Solé A, Diarce G, Haussmann T, Fois M, et al. Intercomparative tests on phase change materials characterisation with differential scanning calorimeter. Appl Energy. 2013;109:415–20.
Grosso R, Matos J, Muccillo E. Thermal and spectroscopic characterization of nanostructured zirconia–scandia–dysprosia. J Therm Anal Calorim. 1–6.
Brill T, Gongwer P, Williams G. Thermal decomposition of energetic materials. 66. Kinetic compensation effects in HMX, RDX, and NTO. J Phys Chem. 1994;98(47):12242–7.
Fathollahi M, Mohammadi B, Mohammadi J. Kinetic investigation on thermal decomposition of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) nanoparticles. Fuel. 2013;104:95–100.
Liu R, Zhou Z, Yin Y, Yang L, Zhang T. Dynamic vacuum stability test method and investigation on vacuum thermal decomposition of HMX and CL-20. Thermochim Acta. 2012;537:13–9.
Křižanovský L, Mentlik V. The use of thermal analysis to predict the thermal life of organic electrical insulating materials. J Therm Anal Calorim. 1978;13(3):571–80.
Eroğlu MS. Thermoanalytical life time testing of energetic poly (glycidyl azide) and its precursor, poly (epichlorodydrin). Polym Bull. 1998;41(1):69–76.
Yi J-H, Zhao F-Q, Wang B-Z, Liu Q, Zhou C, Hu R-Z, et al. Thermal behaviors, nonisothermal decomposition reaction kinetics, thermal safety and burning rates of BTATz-CMDB propellant. J Hazard Mater. 2010;181(1):432–9.
de Klerk W, vander Meer N, Eerligh R. Microcalorimetric study applied to the comparison of compatibility tests (VST and IST) of polymers and propellants. Thermochimica acta. 1995;269:231–43.
Chovancová M, Zeman S. Study of initiation reactivity of some plastic explosives by vacuum stability test and non-isothermal differential thermal analysis. Thermochim Acta. 2007;460(1):67–76.
Zeman S, Gazda Š, Štolcová A, Dimun A. Dependence on temperature of the results of the vacuum stability test for explosives. Thermochim Acta. 1994;247(2):447–54.
Corwin AH, Reinheimer JD. The reduction of carbohydrazide. The acidity of carbohydrazide, semicarbazide and urea1. J Am Chem Soc. 1951;73(3):1184–6.
Rahn PC, Siggia S. Carbohydrazide as a solid reducing agent for reaction gas chromatography. Determination of azo, nitro, and sulfonate compounds. Anal Chem. 1973;45(14):2336–41.
Akiyoshi M, Nakamura H, Hara Y. The thermal behavior of the zinc complexes as a non-azide gas generant for safer driving—Zn complexes of the carbohydrazide and semicarbazide. Propell Explos Pyrot. 2000;25(1):41–6.
Dutta R, Sarkar A. A study of metal complexes of carbohydrazide. J Inorg Nucl Chem. 1981;43(10):2557–9.
Mansour AK, Eid MM, Khalil NS. Synthesis and reactions of some new heterocyclic carbohydrazides and related compounds as potential anticancer agents. Molecules. 2003;8(10):744–55.
Kon’kova T, Matyushin Y, Sidnitskii V. Thermodynamics of coordination Co (II), Ni (II), Zn, and Cd compounds with carbohydrazide. Chem Phys Rep. 1995;14(6):865.
Akiyoshi M, Hirata N, Nakamura H, Hara Y. The thermal behavior of the carbohydrazide complexes of certain metals (1): The synthesis and the thermal analysis. Kayak Gakkaishi. 1996;57(6):238–43.
Bustos C, Burckhardt O, Schrebler R, Carrillo D, Arif A, Cowley A, et al. Synthesis, characterization, and electrochemistry of cis-dioxomolybdenum (VI) complexes of Schiff bases derived from carbohydrazide, thiocarbohydrazide, and salicylaldehyde. Crystal structures of [MoO2 (o-OC6H4CH: NN: CSNHN: CHC6H4OH-o) Me2SO] and [(MoO2)2 (o-OC6H4CH: NN: CONN: CHC6H4O-o)(Me2SO)2]. cntdot. 0.5 Me2SO. Inorg Chem. 1990;29(20):3996–4001.
Bushuyev OS, Arguelles FA, Brown P, Weeks BL, Hope-Weeks LJ. New energetic complexes of copper (II) and the acetone carbohydrazide schiff base as potential flame colorants for pyrotechnic mixtures. Eur J Inorg Chem. 2011;2011(29):4622–5.
Ivanov M, Kalinichenko I. Some complexes of metal nitrates, sulfates and chlorides with carbohydrazide. Russ J Inorg Chem. 1981;26(8):2134–7.
Ivanov M, Kalinichenko I, Savitskii A. Complexes of manganese (II), cobalt (II), nickel (II), cadmium and zinc with carbohydrazide. Russ J Coord Chem. 1985;11(1):45–8.
Sinditskii V, Fogelzang A, Dutov M, Sokol V, Serushkin V, Svetlov B, et al. Structure of complex compounds of metal chlorides, sulfates, nitrates and perchlorates with carbohydrazide. Zh Neorg Khim (Russ J Inorg Chem). 1987;32(8):1944–9.
Sindiskii V, Vernidub TY, Fogelzang A. Metal azide complexes with carbohydrazide. Zn Neorgan Khim (Russ J Inorg Chem). 1990;35(3):685–8.
Sinditskii V, Serushkin V. Design and combustion behaviour of explosive coordination compounds. Def Sci J (Def Sci J). 1996;46(5):371–83.
Akiyoshi M, Hirata N, Nakamura H, Hara Y. The thermal behavior of the carbohydrazide complexes of certain metals (3): the gas evolved in the decomposition of the Mg complex. Kayak Gakkaishi. 1997;58(2):68–75.
Akiyoshi M, Nakamura H, Hara Y. The thermal behavior of the zinc complexes as a non-azide gas generant for safer driving—zn complexes of the carbohydrazide and semicarbazide. Propellants Explos Pyrotech. 2000;25(1):41–6.
Akiyoshi M, Nakamura H, Hara Y. The strontium complex nitrates of carbohydrazide as a non-azide gas generator for safer driving–the thermal behavior of the sr complex with various oxidizing agents. Propellants Explos Pyrotech. 2000;25(5):224–9.
Talawar M, Agrawal A, Chhabra J, Asthana S. Studies on lead-free initiators: synthesis, characterization and performance evaluation of transition metal complexes of carbohydrazide. J Hazard Mater. 2004;113(1):57–65.
Sonawane S, Gore G, Polke B, Nazare A, Asthana S. Transition metal carbohydrazide nitrates: burn-rate modifiers for propellants. Def Sci J. 2006;56(3):391–8.
Wei Z, Zhang T, Lu C, Yu K. A study of preparation and molecular structure of [Cd (NH2NHCONHNH2)(3)](ClO4)(2). Chin J Inorg Chem. 1999;15(4):482–6.
Qi S, Li Z, Zhang T, Zhou Z, Yang L, Zhang J, et al. Crystal structure, thermal analysis and sensitivity property of Zn(CHZ)3(ClO4)2. Acta Chim Sin. 2011;69(8):987–92.
Huang H, Zhang T, Zhang J, Wang L. A screened hybrid density functional study on energetic complexes: cobalt, nickel and copper carbohydrazide perchlorates. J Hazard Mater. 2010;179(1–3):21–7.
Sun Y, Zhang T, Zhang J, Qiao X, Yang L. Flash pyrolysis study of zinc carbohydrazide perchlorate using T-jump/FTIR spectroscopy. Combust Flame. 2006;145(3):643–6.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29(11):1702–6.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38(11):1881–6.
Nong W, Chen X, Wang L, Liang J, Zhong L, Tong Z. Nonisothermal decomposition kinetics of abietic acid in argon atmosphere. Ind Eng Chem Res. 2011;50(24):13727–31.
Chen Z, Chai Q, Liao S, He Y, Li Y, Wu W, et al. Application of simplified version of advanced isoconversional procedure in non-isothermal kinetic study. J Therm Anal Calorim. 2013;113(2):649–57.
Jankovic B. Kinetic analysis of the nonisothermal decomposition of potassium metabisulfite using the model-fitting and isoconversional (model-free) methods. Chem Eng J. 2008;139(1):128–35.
Huang M-X, Zhou C-R, Han X-W. Investigation of thermal decomposition kinetics of taurine. J Therm Anal Calorim. 2013;113(2):589–93.
Jankovic B, Mentus SP. A kinetic study of the nonisothermal decomposition of palladium acetylacetonate investigated by thermogravimetric and x-ray diffraction analysis determination of distributed reactivity model. Metall Mater Trans a-Phys Metall Mater Sci. 2009;40A(3):609–24.
Wada T, Koga N. Kinetics and mechanism of the thermal decomposition of sodium percarbonate: role of the surface product layer. J Phys Chem A. 2013;117(9):1880–9.
Hu RZ, Chen SP, Gao SL, Zhao FQ, Luo Y, Gao HX, et al. Thermal decomposition kinetics of the Pb0.25Ba0.75(TNR)center dot H2O complex. J Hazard Mater. 2005;117(2–3):103–10.
Xu K-Z, Zhang H, Liu P, Huang J, Ren Y-H, Wang B-Z, et al. Structural and thermal characterization of a novel high nitrogen energetic material: (NH4)2DNMT. Propellants Explos Pyrotech. 2012;37(6):653–61.
Ren Y-H, Li W, Zhao F-Q, Yi J-H, Yan B, Ma H-X, et al. Crystal structure and thermal behaviors for 3,5-dinitrobenzoic acid of 3,5-diamino-1,2,4-triazole. J Anal Appl Pyrol. 2013;102:89–96.
Dong H, Hu R, Yao P, Zhang X. Thermograms of energetic materials. Beijing: Natl Def Ind Press; 2002. p. 276–300.
Holl G, Klapotke TM, Polborn K, Rienacker C. Structure and bonding in 2-diazo-4,6-dinitrophenol (DDNP). Propellants Explos Pyrotech. 2003;28(3):153–6.
Yang Z-W, Liu Y-C, Liu D-C, Yan L-W, Chen J. Synthesis and characterization of spherical 2-diazo-4,6-dinitrophenol (DDNP). J Hazard Mater. 2010;177(1–3):938–43.
Xu K, Song J, Zhao F, Ma H, Gao H, Chang C, et al. Thermal behavior, specific heat capacity and adiabatic time-to-explosion of G(FOX-7). J Hazard Mater. 2008;158(2–3):333–9.
Liu R, Zhang T, Yang L, Zhou Z, Hu X. Research on thermal decomposition of trinitrophloroglucinol salts by DSC, TG and DVST. Cent Eur J Chem. 2013;11(5):774–81.
Y-h Sun, Zhang T-L, Zhang J-G, Yang L. The flash pyrolysis process of [Zn (CHZ)3](CIO4)2 monitored with T-jump/FTIR spectroscopy. Initiat Pyrotech. 2005;3(104):18.
Hu R-Z, Shi Q-Z. Thermal analysis kinetics, vol. 56. Beijing: Science Press; 2001. p. 67.
Acknowledgements
This work was supported by Technology fund on Applied Physical Chemistry Laboratory of China (9140C3703051105 and 9140C370303120C37142) and State Key Laboratory of Explosion Science and Technology (QNKT12-02).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Jiang, YT., Zhang, TL. et al. Thermal kinetic performance and storage life analysis of a series of high-energy and green energetic materials. J Therm Anal Calorim 119, 659–670 (2015). https://doi.org/10.1007/s10973-014-4180-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4180-x