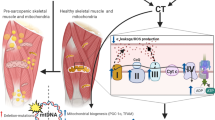
Abstract
Mitochondria are essential energy-providing organelles that are required in the maintenance of healthy skeletal muscle. As such, the removal of damaged mitochondria, through mitophagy, is necessary to maintain mitochondrial quality. In aging muscle, mitochondrial content and function are often found to be reduced compared to young individuals. This occurs despite the fact that measures of mitophagy are elevated, suggesting that mitophagy is insufficiently high to remove all of the dysfunctional organelles in aging muscle. Recent evidence has shown that acute exercise promotes mitophagic signaling, leading to organelle degradation. This exercise-induced signaling is attenuated in aging muscle, suggesting that aging muscle loses its capacity for mitochondrial turnover in response to exercise. This contributes to the reduction in muscle health in elderly individuals. Chronic exercise training improves mitochondrial content and function, even in aging muscle, leading to reduced mitophagy signaling. Thus, exercise training should be prescribed for both young and elderly populations to promote the maintenance of a healthy mitochondrial pool, through the stimulation of both organelle biogenesis and mitophagy.

Similar content being viewed by others
Abbreviations
- AMP:
-
Adenosine monophosphate
- AMPK:
-
AMP-activated protein kinase
- LC3:
-
Microtubule-associated proteins 1A/1B light chain 3A
- LAMP 1/2:
-
Lysosomal-associated membrane proteins 1 and 2
- MIT/TFE:
-
Microthalmia-transcription factor E
- mTORC1:
-
Mammalian/mechanistic target of rapamycin complex 1
- NUGEMP:
-
Nuclear gene-encoded mitochondrial protein
- PGC-1α:
-
Peroxisome proliferator activator receptor (PPAR) coactivator 1 alpha
- PINK1:
-
PTEN-induced putative kinase 1
- p38MAPK:
-
p38 mitogen-activated protein kinase
- TFEB:
-
Transcription factor EB
- ULK1:
-
Serine/threonine protein kinase
References
Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1α transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem. 2005;280(20):19587–93. https://doi.org/10.1074/jbc.M408862200.
Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16(14):1879–86. https://doi.org/10.1096/fj.02-0367com.
Brandt N, Gunnarsson TP, Bangsbo J, Pilegaard H. Exercise and exercise training-induced increase in autophagy markers in human skeletal muscle. Physiol Rep. 2018;6(7):1–12. https://doi.org/10.14814/phy2.13651.
Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. AJP Cell Physiol. 2004;287(4):C817–33. https://doi.org/10.1152/ajpcell.00139.2004.
Cantó C, Gerhart-hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliot PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2013;458(7241):1056–60. https://doi.org/10.1038/nature07813.
Carter HN, Chen CCW, Hood DA. Mitochondria, muscle health, and exercise with advancing age. Physiology. 2015;30(3):208–23. https://doi.org/10.1152/physiol.00039.2014.
Carter HN, Kim Y, Erlich AT, Zarrin-Khat D, Hood DA. Autophagy and mitophagy flux in young and aged skeletal muscle following chronic contractile activity. J Physiol. 2018;596(16):3567–84. https://doi.org/10.1113/JP275998.
Chapin HC, Okada M, Merz AJ, Miller DL. Tissue-specific autophagy responses to aging and stress in C. elegans. Aging (Albany NY). 2015;7(6):419–34. https://doi.org/10.18632/aging.100765.
Chen CCW, Erlich AT, Crilly MJ, Hood DA. Parkin is required for exercise-induced mitophagy in muscle: impact of aging. Am J Physiol Metab ajpendo. 2018;315(3):E404-E415. https://doi.org/10.1152/ajpendo.00391.2017.
Chen CCW, Erlich AT, Hood DA. Role of Parkin and endurance training on mitochondrial turnover in skeletal muscle. Skelet Muscle. 2018;8(1):1–14. https://doi.org/10.1186/s13395-018-0157-y.
Drake JC, Wilson RJ, Yan Z. Molecular mechanisms for mitochondrial adaptation to exercise training in skeletal muscle. FASEB J. 2016;30(1):13–22. https://doi.org/10.1096/fj.15-276337.
Erlich AT, Brownlee DM, Beyfuss K, Hood DA. Exercise induces TFEB expression and activity in skeletal muscle in a PGC-1α-dependent manner. Am J Physiol Physiol. 2018;314(1):C62–72. https://doi.org/10.1152/ajpcell.00162.2017.
Eskelinen EL. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol Aspects Med. 2006;27(5–6):495–502. https://doi.org/10.1016/j.mam.2006.08.005.
Geisler S, Holmström KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12(2):119–31. https://doi.org/10.1038/ncb2012.
He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun QM Korsmeyer S, Packer M, May HI, Hill JA, Virgin HW, Gilpin C, Xiao G, Bassel-Duby R, Scherer PE, Levine B. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481(7382):511–5. https://doi.org/10.1038/nature10758.
Hepple RT. Mitochondrial involvement and impact in aging skeletal muscle. Front Aging Neurosci. 2014;6:1–13. https://doi.org/10.3389/fnagi.2014.00211.
Hood DA. Invited review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol. 2001;90(3):1137–57.
Hood DA. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl Physiol Nutr Metab. 2009;34(3):465–72. https://doi.org/10.1139/H09-045.
Hood DA, Memme JM, Oliveira AN, Triolo M. Maintenance of skeletal muscle mitochondria in health, exercise, and aging. Annu Rev Physiol. 2019;81:19–41. https://doi.org/10.1146/annurev-physiol-020518-114310.
Hood DA, Uguccioni G, D’Souza D. Regulation of PPARγ coactivator-1 function and expression in muscle: effect of exercise. PPAR Res. 2010. https://doi.org/10.1155/2010/937123.
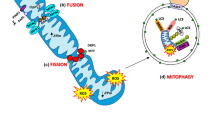
Iqbal S, Hood DA. The role of mitochondrial fusion and fission in skeletal muscle function and ysfunction. Front Biosci. 2015;20:57–172.
Iqbal S, Ostojic O, Singh K, Joseph A-M, Hood DA. Expression of mitochondrial fission and fusion regulatory proteins in skeletal muscle during chronic use and disuse. Muscle Nerve. 2013;48(6):963–70. https://doi.org/10.1002/mus.23838.
Jamart C, Naslain D, Gilson H, Francaux M. Higher activation of autophagy in skeletal muscle of mice during endurance exercise in the fasted state. AJP Endocrinol Metab. 2013;305(8):E964–74. https://doi.org/10.1152/ajpendo.00270.2013.
Ju JS, Jeon SI, Park JY, Lee JY, Lee SC, Cho KJ, Jeong JM. Autophagy plays a role in skeletal muscle mitochondrial biogenesis in an endurance exercise-trained condition. J Physiol Sci. 2016;66(5):417–30. https://doi.org/10.1007/s12576-016-0440-9.
Kang S-H, Lee H-A, Kim M, Lee E, Sohn UD, Kim I. Forkhead box O3 plays a role in skeletal muscle atrophy through expression of E3 ubiquitin ligases MuRF-1 and atrogin-1 in Cushing’s syndrome. Am J Physiol Endocrinol Metabol. 2017;312(6):E495–507. https://doi.org/10.1152/ajpendo.00389.2016.
Kim Y, Hood DA. Regulation of the autophagy system during chronic contractile activity-induced muscle adaptations. Physiol Rep. 2017;5(14):1–11. https://doi.org/10.14814/phy2.13307.
Kim Y, Triolo M, Hood DA. Impact of aging and exercise on mitochondrial quality control in skeletal muscle. Oxid Med Cell Longev. 2017. https://doi.org/10.1155/2017/3165396.
Kim Y, Triolo M, Erlich AT, Hood DA. Regulation of autophagic and mitophagic flux during chronic contractile activity-induced muscle adaptations. Pflugers Arch Eur J Physiol. 2018;471(3):431–40. https://doi.org/10.1007/s00424-018-2225-x.
Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Arozena AA, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016;12(1):1–222. https://doi.org/10.1080/15548627.2015.1100356.
Laker RC, Drake JC, Wilson RJ, Lira VA, Lewellen BM, Ryall KA, Fischer CC, Zhand M, Saucerman JJ, Goodyear LJ, Kundu M, Yan Z. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat Commun. 2017;8(1):548. https://doi.org/10.1038/s41467-017-00520-9.
Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122(Pt 20):3589–94. https://doi.org/10.1242/jcs.051011.
Leduc-Gaudet J-P, Reynaud O, Hussain SN, Gouspillou G. Parkin overexpression protects from aging-related loss of muscle mass and strength. J Physiol. 2019;597(7):1975–91. https://doi.org/10.1113/JP277157.
Lee Y, Lee H-Y, Hanna RA, Gustafsson AB. Mitochondrial autophagy by Bnip3 involves Drp1-mediated mitochondrial fission and recruitment of Parkin in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2011;301(5):H1924–31. https://doi.org/10.1152/ajpheart.00368.2011.
Lira VA, Okutsu M, Zhang M, Greene NP, Laker RC, Breen DS, Hoehn KL, Yan Z. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J. 2013;27(10):4184–93. https://doi.org/10.1096/fj.13-228486.
Mansueto G, Armani A, Viscomi C, D’Orsi L, De Cegli R, Polishchuk EV, Lamperti C, Di Meo I, Romanello V, Marchet S, Saha PK, Zong H, Blaauw B, Solagna F, Teeze C, Grumati P, Bonaldo P, Pessin JE, Zeviani M, Sandri M, Ballabio A. transcription factor EB controls metabolic flexibility during exercise. Cell Metab. 2017;25(1):182–96. https://doi.org/10.1016/j.cmet.2016.11.003.
Marcil M, Bourduas K, Ascah A, Burelle Y. Exercise training induces respiratory substrate-specific decrease in Ca2+-induced permeability transition pore opening in heart mitochondria. AJP Hear Circ Physiol. 2006;290(4):H1549–57. https://doi.org/10.1152/ajpheart.00913.2005.
Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, Kimura M, Komatsu M, Hattori N, Tanaka K. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189(2):211–21. https://doi.org/10.1083/jcb.200910140.
Medina DL, Ballabio A. Lysosomal calcium regulates autophagy. Autophagy. 2015;11(6):970–1. https://doi.org/10.1080/15548627.2015.1047130.
Medina DL, Paola S Di, Peluso I, Armani A, De Stefani D, Venditti R, Montefusco S, Scotto-Rosato A, Prezioso C, Forrester A, Settembre C, Wang W, Gao Q, Xu H, Sandri M, Rizzuto R, De Matteis MA, Ballabio A. Lysosomal calcium signaling regulates autophagy via calcineurin and TFEB. Nat Cell Biol. 2015;17(3):288–99. https://doi.org/10.1038/ncb3114.Lysosomal.
Napolitano G, Ballabio A. TFEB at a glance. J Cell Sci. 2016;129(13):2475–81. https://doi.org/10.1242/jcs.146365.
O’Leary MF, Vainshtein A, Iqbal S, Ostojic O, Hood DA. Adaptive plasticity of autophagic proteins to denervation in aging skeletal muscle. Am J Physiol Cell Physiol. 2013;304(5):C422–30.
Palmieri M, Impey S, Kang H, Di Ronza A, Pelz C, Sardiello M, Ballabio A. Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum Mol Genet. 2011;20(19):3852–66. https://doi.org/10.1093/hmg/ddr306.
Pilegaard H, Saltin B, Neufer DP. Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J Physiol. 2003;546(3):851–8. https://doi.org/10.1113/jphysiol.2002.034850.
Pogozelski AR, Geng T, Li P, Yin X, Lira VA, Zhang M, Chi JT, Yan Z. P38 mitogen-activated protein kinase is a key regulator in skeletal muscle metabolic adaptation in mice. PLoS One. 2009;4(11):e7934. https://doi.org/10.1371/journal.pone.0007934.
Roczniak-ferguson A, Petit CS, Froehlich F, Qian S, Ky J, Angarola B, Walther TC, Feguson SM. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci Signal. 2012;5(228):ra42. https://doi.org/10.1126/scisignal.2002790.
Romanello V, Sandri M. Mitochondrial quality control and muscle mass maintenance. Front Physiol. 2016;6:1–21. https://doi.org/10.3389/fphys.2015.00422.
Russell AP, Foletta VC, Snow RJ, Wadley GD. Skeletal muscle mitochondria: a major player in exercise, health and disease. Biochim Biophys Acta Gen Subj. 2014;1840(4):1276–84. https://doi.org/10.1016/j.bbagen.2013.11.016.
Safdar A, Little JP, Stokl AJ, Hettinga BP, Akhtar M, Tarnopolsky MA. Exercise increases mitochondrial PGC-1α content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. J Biol Chem. 2011;286(12):10605–17. https://doi.org/10.1074/jbc.M110.211466.
Sandri M. Autophagy in skeletal muscle. FEBS Lett. 2010;584(7):1411–6. https://doi.org/10.1016/j.febslet.2010.01.056.
Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2013;117(3):399–412.
Settembre C, Ballabio A. TFEB regulates autophagy: an integrated coordination of cellular degradation and recycling processes. Autophagy. 2011;7(11):1379–81. https://doi.org/10.4161/auto.7.11.17166.
Settembre C, Zoncu R, Medina DL, Vetrini F, Erdin S, Huynh T, Ferron M, Karsenty G, Vellard MC, Facchinetti V, Sabatini DM, Ballabio A. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 2012;31(5):1095–108. https://doi.org/10.1038/emboj.2012.32.
Settembre C, De Cegli R, Mansueto G, Saha PK, Vetrini F, Visvikis O, Huynh T, Carissimo A, Palmer D, Klisch TJ, Wollenberg AC, Di Bernardo D, Chan L, Irazoqui JE, Ballabio A. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat Cell Biol. 2013;15(6):647–58. https://doi.org/10.1038/ncb2718.TFEB.
Tai H, Wang Z, Gong H, Han X, Zhou J, Wang X, Wei X, Ding Y, Huang N, Qin J, Zhang J, Wang S, Gao F, Chrzanowska-Lightowlers ZM, Xiang R, Xiao H. Autophagy impairment with lysosomal and mitochondrial dysfunction is an important characteristic of oxidative stress-induced senescence. Autophagy. 2017;13(1):99–113.
Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, Youle RJ. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol. 2010;191(7):1367–80. https://doi.org/10.1083/jcb.201007013.
Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol. 2004;36(12):2503–18. https://doi.org/10.1016/j.biocel.2004.05.009.
Triolo M, Hood DA. Mitochondrial breakdown in skeletal muscle and the emerging role of the lysosomes. Arch Biochem Biophys. 2019;661:66–73. https://doi.org/10.1016/j.abb.2018.11.004.
Vainshtein A, Hood DA. The regulation of autophagy during exercise in skeletal muscle. J Appl Physiol. 2016;120(6):664–73. https://doi.org/10.1152/japplphysiol.00550.2015.
Vainshtein A, Tryon LD, Pauly M, Hood DA. Role of PGC-1α during acute exercise-induced autophagy and mitophagy in skeletal muscle. Am J Physiol Cell Physiol. 2015;308(9):C710–9. https://doi.org/10.1152/ajpcell.00380.2014.
Vincow ES, Merrihew G, Thomas RE, Shulman NJ, Beyer RP, MacCoss MJ, Pallanck LJ. The PINK1-Parkin pathway promotes both mitophagy and selective respiratory chain turnover in vivo. Proc Natl Acad Sci. 2013;110(16):6400–5. https://doi.org/10.1073/pnas.1221132110.
Wei H, Liu L, Chen Q. Selective removal of mitochondria via mitophagy: distinct pathways for different mitochondrial stresses. Biochim Biophys Acta Mol Cell Res. 2015;1853(10):2784–90. https://doi.org/10.1016/j.bbamcr.2015.03.013.
Yang M, Liu E, Tang L, Lei Y, Sun X, Hu J, Dong H, Yang SM, Gao M. Tang B. Emerging roles and regulation of MiT/TFE transcriptional factors. Cell Commun Signal. 2018;16(31):1–11. https://doi.org/10.1186/s12964-018-0242-1.
Yoo S, No M, Heo J, Park D, Kang J, Kim SH, Kwak H. Role of exercise in age-related sarcopenia. J Exerc Rehabil. 2018;14(1):551–8.
Yoshii SR, Mizushima N. Monitoring and measuring autophagy. Int J Mol Sci. 2017;18(9):1–13. https://doi.org/10.3390/ijms18091865.
Zhou J, Tan SH, Nicolas V, Bauvy C, Yang ND, Zhang J, Xue Y, Codogno P, Shen HM. Activation of lysosomal function in the course of autophagy via mTORC1 suppression and autophagosome-lysosome fusion. Cell Res. 2013;23(4):508–23. https://doi.org/10.1038/cr.2013.11.
Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the Vacuolar H+-ATPase. Science. 2011;334(6056):678–83. https://doi.org/10.1126/science.1207056.mTORC1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Erlich, A.T., Hood, D.A. Mitophagy Regulation in Skeletal Muscle: Effect of Endurance Exercise and Age. J. of SCI. IN SPORT AND EXERCISE 1, 228–236 (2019). https://doi.org/10.1007/s42978-019-00041-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42978-019-00041-5