Abstract
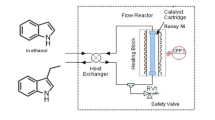
A two-step flow reactor has been set up for an expeditious synthesis of 4-benzylidene-pyrazol-5-one derivatives. This procedure involved cyclization of aromatic hydrazine/β-keto ester and subsequent Knoevenagel condensation reaction with aromatic aldehydes in tandem without isolation of intermediates. Residence time was reduced to less than 2 min in total through conducting this reaction at a relatively high temperature. Accordingly, 15 samples were achieved and isolated in moderate to excellent yields.

ᅟ








Similar content being viewed by others
References
A. Weissberger. In: Wiley, R. H., Wiley, P. (eds.) The Chemistry of Heterocyclic Compounds: Pyrazolinones, Pyrazolidones and Derivatives. Wiley, New York; 1964
S. Scheibye, A. A. El-Barbary, S. O. Lawesson, H. Fritz, G. Rihs. Tetrahedron 1982; 38, 3753
Y. Kakiuchi, N. Sasaki, M. Satoh-Masuoka, H. Murofushi, Murakami-Murofushi, K. Biochem. Biophys. Res. Commun. 2004; 320, 1351
S. P. Hiremath, K. Rudresh, A. R. Saundane. Indian J. Chem. 2002; 41B, 394
In: H. A. Lubs (ed.) The Chemistry of Synthetic Dyes and Pigments. American Chemical Society, Washington, DC; 1970
Joerg S, Reinhold G, Joachim OS, Robert S, Klaus L, Offen G (1988) Chem Abstr 108:167465 DE3, 625
A. B. Uzoukwu. Polyhedron 1993; 12, 2719
G. Desimoni, L. Astolfi, M. Cambieri, A. Gamber, G. Tacconi. Tetrahedron 1973; 29, 2627
W. S. Hamama. Synth. Commun. 2001; 31, 1335
M. M. Mojtahedi, M. R. Jalali, M. S. Abaee, M. Bolourtchian. Heterocycl. Commun. 2006; 12, 225
B. R. Vaddula, R. S. Varma, J. Leazer. Tetrhedron Lett. 2013; 54, 1538
M. M. Mojtahedi, M. Javadpour, M. S. Abaee. Ultrason. Sonochem. 2008; 15, 828
M. X. Guo, J. X. Guo, D. Z. Jia, H. Liu, L. Liu, A. J. Liu, F. Li. J. Mol. Struct. 2013; 1035, 271
Ahmad N (2011) Acta Ciencia Indica. Chemistry 37:5
E. Garcia-Egido, S. Y. Wong, B. H. Warrington. Lab Chip 2002; 2, 31
M. Fernandez-Suarez, S. Y. Wong, B. H. Warrington. Lab Chip 2002; 2, 170
E. Garcia-Egido, V. Spikmans, S. Y. Wong, B. H. Warrington. Lab Chip 2003; 3, 73
D. Obermayer, T. N. Glasnov, C. O. Kappe. J. Org. Chem. 2011; 76, 6657
J. Pelleter, F. Renaud. Org. Process. Res. Dev. 2009; 13, 698
M. S. K. Youssef, S. A. M. Metwally, M. A. El-Mahraby, M. I. Younes. J. Heterocyclic Chem. 1984; 21, 1747
P. Sun, D. Yang, W. Wei, X. Sun, W. Zhang, H. Zhang, Y. Wang, H. Wang. Tetrahedron 2017; 73, 2022
V. Hessel (2009). Chem. Eng. Technol. 32, 1655
B. Wahab, G. Ellames, S. Passey, P. Watts (2010). Tetrahedron 66, 3861
E. Riva, S. Gagliardi, C. Mazzoni, D. Passarella, A. Rencurosi, D. Vigo, A. Rencurosi (2010). Tetrahedron 66, 3242
Z. Q. Yu, Y. W. Lv, C. M. Yu (2012). Org. Process. Res. Dev. 16, 1669
J. Wegner, S. Ceylan, A. Kirschning. Adv. Synth. Catal. 2012; 354, 17
C. Wiles, P. Watts. Green Chem. 2012; 14, 38
Z. Q. Yu, Y. W. Lv, C. M. Yu, W. K. Su. Tendrahedron Lett. 2013; 54, 1261
Z. Q. Yu, Y. W. Lv, C. M. Yu, W. K. Su. Org. Process Res. Dev. 2013; 17, 438
C. Wiles, P. Watts. Green Chem. 2014; 16, 55
D. Cambié, C. Bottecchia, N. J. W. Straathof, V. Hessel and T. Noël. Chem. Rev. 2016; 116, 10276
B. Gutmann, D. Cantillo and C. O. Kappe. Angew. Chem. Int. Ed. 2015; 54, 6688–6728
J. Britton. J. Flow Chem. 2016; 6, 123
S. Chada, D. Mandala and P. Watts. J. Flow Chem. 2017; 7, 37
Y. W. Lv, Z. Q. Yu, W. K. Su. Org. Process. Res. Dev. 2011; 15, 471
J. Yu, J. Xu, Z. Yu, Y. Jin, J. Li, Y. Lv. J. Flow Chem. 2017; 7, 33
J. Xu, J. Yu, Y. Jin, J. Li, Z. Yu, Y. Lv. Chem. Eng. Process 2017; 121, 144
W. P. Bula, W. Verboom, D. N. Reinhoudt, H. J. G. E. Gardeniers. Lab Chip 2007; 7, 1717
E. G. Moschetta, S. Negretti, K. M. Chepiga, N. A. Brunelli, Y. Labreche, Y. Feng, F. Rezaei, R. P. Lively, W. J. Koros, H. M. L. Davies, C. W. Jones. Angew. Chem. Int. Ed. 2015; 54, 6470
M. Lopez-Pastor, A. Dominguez-Vidal, M. J. Ayora-Canada, T. Laurell, M. Valcarcel, B. Lendl. Lab Chip 2007; 7, 126
H. Seyler, S. Haid, T.-H. Kwon, D. J. Jones, P. Baeuerle, A. B. Holmes, W. W. H. Wong. Aust. J. Chem. 2013; 66, 151
V. Pandarus, G. Gingras, F. Beland, R. Ciriminna, M. Pagliaro. Catal. Sci. Technol. 2011; 1, 1600
M. Tarleton, A. McCluskey. Tetrahedron Lett. 2011; 52, 1583
N. Nikbin, P. Watts. Org. Process. Res. Dev. 2004; 8, 942
R. Munirathinam, J. Huskens, W. Verboom. Adv. Synth. Catal. 2015; 357, 1093
K. Suzdalev, M. Babakova. Russ. J. Org. Chem. 2005; 41, 233
Z. Han, X. Liang, Y. Wang, J. Qing, L. Cao, L. Shang, Z. Yin. Eur. J. Med. Chem. 2016; 116, 147
R. Ramajayam, K.-P. Tan, H.-G. Liu, P.-H. Liang, Bioorg. Med. Chem. 2010; 18, 7849
M. Parveen, S. Azaz, A. M. Malla, F. Ahmad, M. Ahmad, M. Gupta. RSC Adv. 2016; 6, 148
Acknowledgments
Financial support from the National Science Foundation of China (21476128) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 19562 kb)
Rights and permissions
About this article
Cite this article
Yu, J., Xu, J., Li, J. et al. A continuous-flow procedure for the synthesis of 4-Benzylidene-pyrazol-5-one derivatives. J Flow Chem 8, 29–34 (2018). https://doi.org/10.1007/s41981-018-0003-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41981-018-0003-8