Abstract
Currently, Fe-N-C materials are considered to be among the most important oxygen reduction reaction (ORR) catalysts, because they are potential substitutes for Pt-based catalysts and are therefore promising in the development of non-noble metal-based catalysts. However, challenges such as electron transfer kinetics still exist and need to be improved upon. From a chemical stand point, improvements can be made through the better understanding of mechanisms in Fe-N-C-based ORR catalysis along with a deeper understanding of the chemical origin of active sites on Fe-N-C catalyst surfaces. Based on these, this comprehensive review will focus on the energy conversion, transformation kinetics and electron transfer of the ORR process as catalyzed by Fe-N-C catalysts. And by taking these and other relevant analytical results for Fe-N-C materials into consideration, primary strategies in the improvement in Fe-N-C catalyst activity will be presented.
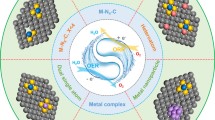
Graphical Abstract
As the promising Pt substrate for oxygen reduction catalysis, the Fe-N-C materials are active toward the four-electron reduction of O2 to H2O. This review focuses on the profound understanding of heterogeneous oxygen reduction reaction on Fe-N-C materials from the following aspects: (1) thermodynamics of energy conversion in ORR processes, (2) kinetics of ORR processes based on Fe-N-C catalysts, (3) the textural features of Fe-N-C and analytic results known as far, (4) fundamental principle for Fe-N-C materials synthesis and (5) practical application for fuel cell and metal–air batteries.





(Part a, b and c adapted with permission from Ref. [35]; Copyright 2013 American Chemical Society.)


(Part a, b, c, d, e and f adapted with permission from Ref. [60]; Copyright 2016 American Chemical Society.)

(Part a adapted with permission from Ref. [48]; Copyright 2017 Elsevier. Part b adapted with permission from Ref. [41]; Copyright 2014 American Chemical Society. Part c adapted with permission from Ref. [6]; Copyright 2016 American Chemical Society. Part d adapted with permission from Ref. [104]; Copyright 2015 American Chemical Society.)

(Part a adapted with permission from Ref. [115]; Copyright 2016 John Wiley and Sons. Part b adapted with permission from Ref. [116]; Copyright 2017 American Chemical Society. Part c adapted with permission from Ref. [76]; Copyright 2017 American Chemical Society. Part d adapted with permission from Ref. [56]; Copyright 2017 John Wiley and Sons.)

(Part a adapted with permission from Ref. [144]. Part b adapted with permission from Ref. [107]; Copyright 2009 American Association for the Advancement of Science. Part c adapted with permission from Ref. [145]; Copyright 2018 John Wiley and Sons. Part d adapted with permission from Ref. [146]; Copyright 2011 Nature Publishing Group.)

(Parts a and b adapted with permission from Ref. [152]; Copyright 2018 American Association for the Advancement of Science. Parts c and d adapted with permission from Ref. [161]; Copyright 2018 Elsevier. Part e adapted with permission from Ref. [94]; Copyright 2018 American Chemical Society. Part f adapted with permission from Ref. [162]; Copyright 2018 Royal Society of Chemistry.)
Similar content being viewed by others
References
Wang, Y., Qiu, W., Song, E., et al.: Adsorption-energy-based activity descriptors for electrocatalysts in energy storage applications. National Sci. Rev. 5, 327–341 (2018)
Shao, M., Chang, Q., Dodelet, J.P., et al.: Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 116, 3594–3657 (2016)
Nørskov, J.K., Rossmeisl, J., Logadottir, A., et al.: Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004)
Wang, Y.J., Zhao, N., Fang, B., et al.: Carbon-supported Pt-based alloy electrocatalysts for the oxygen reduction reaction in polymer electrolyte membrane fuel cells: particle size, shape, and composition manipulation and their impact to activity. Chem. Rev. 115, 3433–3467 (2015)
Li, Y., Zhou, W., Wang, H., et al.: An oxygen reduction electrocatalyst based on carbon nanotube-graphene complexes. Nat. Nanotech. 7, 394–400 (2012)
Sa, Y.J., Seo, D.J., Woo, J., et al.: A general approach to preferential formation of active Fe-Nx sites in Fe-N/C electrocatalysts for efficient oxygen reduction reaction. J. Am. Chem. Soc. 138, 15046 (2016)
Liu, Y., Jiang, H., Zhu, Y., et al.: Transition metals (Fe Co, and Ni) encapsulated in nitrogen-doped carbon nanotubes as bi-functional catalysts for oxygen electrode reactions. J. Mater. Chem. A 4, 1694–1701 (2016)
Li, J., Hou, P., Zhao, S., et al.: A 3D bi-functional porous N-doped carbon microtube sponge electrocatalyst for oxygen reduction and oxygen evolution reactions. Energy Environ. Sci. 9, 3079–3084 (2016)
Zhang, J., Dai, L.: Heteroatom-doped graphitic carbon catalysts for efficient electrocatalysis of oxygen reduction reaction. ACS Catal. 5, 7244–7253 (2015)
Tang, C., Zhang, Q.: Nanocarbon for oxygen reduction electrocatalysis: dopants, edges, and defects. Adv Mater 29(13), 1703185 (2017)
Vesborg, P.C.K., Jaramillo, T.F.: Addressing the terawatt challenge: scalability in the supply of chemical elements for renewable energy. RSC Adv. 2, 7933–7947 (2012)
Ren, G., Lu, X., Li, Y., Zhu, Y., et al.: Porous core-shell Fe3C embedded N-doped carbon nanofibers as an effective electrocatalysts for oxygen reduction reaction. ACS Appl. Mater. Interfaces 8, 4118–4125 (2016)
Saha, B., Gupta, D., Abu-Omar, M.M., et al.: Porphyrin-based porous organic polymer-supported iron(III) catalyst for efficient aerobic oxidation of 5-hydroxymethyl-furfural into 2,5-furandicarboxylic acid. J. Catal. 299, 316–320 (2013)
Deng, D., Chen, X., Yu, L., et al.: A single iron site confined in a graphene matrix for the catalytic oxidation of benzene at room temperature. Sci. Adv. 1, 1500462 (2015)
Zhang, P., Chen, X.F., Lian, J.S., et al.: Structural selectivity of CO oxidation on Fe/N/C catalysts. J. Phys. Chem. C 116, 17572–17579 (2012)
Liu, W., Zhang, L., Liu, X., et al.: Discriminating catalytically active FeNx species of atomically dispersed Fe–N–C catalyst for selective oxidation of the C–H bond. J. Am. Chem. Soc. 139, 10790–10798 (2017)
Ju, W., Bagger, A., Hao, G.P., et al.: Understanding activity and selectivity of metal-nitrogen-doped carbon catalysts for electrochemical reduction of CO2. Nat. Commun. 8, 944 (2017)
Huan, T.N., Ranjbar, N., Rousse, G., et al.: Electrochemical reduction of CO2 catalyzed by Fe–N–C materials: a structure-selectivity study. ACS Catal. 7, 1520–1525 (2017)
Xu, S., Yu, D., Liao, S., et al.: Nitrogen-doped carbon supported iron oxide as efficient catalysts for chemoselective hydrogenation of nitroarenes. RSC Adv. 6, 96431–96435 (2016)
Li, J., Jj, Zhang, Liu, H., et al.: Graphitic carbon nitride (g–C3N4)-derived Fe–N–C catalysts for selective hydrodeoxygenation of 5-hydroxymethylfurfural to 2,5-dimethylfuran. ChemistrySelect 2, 11062–11070 (2017)
Nie, Y., Li, L., Wei, Z.: Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem. Soc. Rev. 44, 2168–2201 (2015)
Scofield, M.E., Liu, H., Wong, S.S.: A concise guide to sustainable PEMFCs: recent advances in improving both oxygen reduction catalysts and proton exchange membranes. Chem. Soc. Rev. 44, 5836–5860 (2015)
Raj, C.R., Samanta, A., Noh, S.H., et al.: Emerging new generation electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 4, 11156–11178 (2016)
Xia, W., Mahmood, A., Liang, Z., et al.: Earth-abundant nanomaterials for oxygen reduction. Angew. Chem. Int. Ed. 55, 2650–2676 (2015)
Xia, Z., An, L., Chen, P., et al.: Non-Pt nanostructured catalysts for oxygen reduction reaction: synthesis, catalytic activity and its key factors. Adv. Energy Mater. 6, 1600458 (2016)
Wenling, G., Liuyong, H., Jing, L., et al.: Recent advancements in transition metal-nitrogen–carbon catalysts for oxygen reduction reaction. Electroanalysis 30, 1217–1228 (2018)
Atkins, P.W., De Paula, J.: Atkins’ Physical Chemistry. Oxford University Press, Oxford (2006)
Wan, K., Yu, Z., Li, X., et al.: pH Effect on electrochemistry of nitrogen-doped carbon catalyst for oxygen reduction reaction. ACS Catal. 5, 4325–4332 (2015)
Muthukrishnan, A., Nabae, Y., Okajima, T., et al.: Kinetic approach to investigate the mechanistic pathways of oxygen reduction reaction on Fe-containing N-doped carbon catalysts. ACS Catal. 5, 5194–5202 (2015)
Damjanovic, A., Genshaw, M.A., Bockris, J.O.M.: Distinction between intermediates produced in main and side electrodic reactions. J. Chem. Phys. 45, 4057–4059 (1966)
Muthukrishnan, A., Nabae, Y., Hayakawa, T., et al.: Fe-containing polyimide-based high-performance ORR catalysts in acidic medium: a kinetic approach to study the durability of catalysts. Catal. Sci. Technol. 5, 475–483 (2015)
Wu, J., Zhang, D., Niwa, H., et al.: Enhancement in kinetics of the oxygen reduction reaction on a nitrogen-doped carbon catalyst by introduction of iron via electrochemical methods. Langmuir 31, 5529–5536 (2015)
Wu, K.H., Shi, W., Wang, D., et al.: In situ electrostatic modulation of path selectivity for the oxygen reduction reaction on Fe–N doped carbon catalyst. Chem. Mater. 29, 4649–4653 (2017)
Nayak, S., McPherson, I.J., Vincent, K.A.: Adsorbed intermediates in oxygen reduction on platinum nanoparticles observed by in situ IR spectroscopy. Angew. Chem. Int. Ed. 130, 1–5 (2018)
Ramaswamy, N., Tylus, U., Jia, Q., et al.: Activity descriptor identification for oxygen reduction on nonprecious electrocatalysts: linking surface science to coordination chemistry. J. Am. Chem. Soc. 135, 15443–15449 (2013)
Jia, Q., Ramaswamy, N., Hafiz, H., et al.: Experimental observation of redox-induced Fe–N switching behavior as a determinant role for oxygen reduction activity. ACS Nano 9, 12496–12505 (2015)
Xu, H., Cheng, D., Cao, D., et al.: A universal principle for a rational design of single-atom electrocatalysts. Nat. Catal. 1, 339–348 (2018)
Zitolo, A., Goellner, V., Armel, V., et al.: Identification of catalytic sites for oxygen reduction in iron-and nitrogen-doped graphene materials. Nat. Mater. 14, 937–942 (2015)
Malko, D., Kucernak, A., Lopes, T.: In situ electrochemical quantification of active sites in Fe–N/C non-precious metal catalysts. Nat. Commun. 7, 13285 (2016)
Kong, A., Zhu, X., Han, Z., et al.: Ordered hierarchically micro- and mesoporous Fe–Nx-embedded graphitic architectures as efficient electrocatalysts for oxygen reduction reaction. ACS Catal. 4, 1793–1800 (2014)
Wang, Q., Zhou, Z., Lai, Y., et al.: Phenylenediamine-based FeNx/C catalyst with high activity for oxygen reduction in acid medium and its active-site probing. J. Am. Chem. Soc. 136, 10882–10885 (2014)
Kramm, U.I., Lefèvre, M., Larouche, N., et al.: Correlations between Mass Activity and physicochemical properties of Fe/N/C catalysts for the ORR in PEM fuel cell via 57Fe Mössbauer spectroscopy and other techniques. J. Am. Chem. Soc. 136, 978–985 (2014)
Lefèvre, M., Dodelet, J.P., Bertrand, P.: Molecular oxygen reduction in PEM Fuel Cells: evidence for the simultaneous presence of two active sites in Fe-based catalysts. J. Phys. Chem. B 106, 8705–8713 (2002)
Lai, Q., Zheng, L., Liang, Y., et al.: Metal-organic-framework-derived Fe-N/C electrocatalyst with five-coordinated Fe-Nx sites for advanced oxygen reduction in acid media. ACS Catal. 7, 1655–1663 (2017)
Li, Q., Li, X., Zhang, G., et al.: Cooperative spin transition of monodispersed FeN3 sites within graphene induced by CO adsorption. J. Am. Chem. Soc. 140, 15149–15152 (2018)
Zhu, Y., Zhang, B., Liu, X., et al.: Unravelling the structure of electrocatalytically active Fe-N complexes in carbon for the oxygen reduction reaction. Angew. Chem. Int. Ed. 53, 10673–10677 (2014)
Jaouen, F., Herranz, J., Lefèvre, M., et al.: Cross-laboratory experimental study of non-noble-metal electrocatalysts for the oxygen reduction reaction. ACS Appl. Mater. Interfaces 1, 1623–1639 (2009)
Shen, H., Gracia-Espino, E., Ma, J., et al.: Atomically FeN2 moieties dispersed on mesoporous carbon: a new atomic catalyst for efficient oxygen reduction catalysis. Nano Energy 35, 9–16 (2017)
Jiang, W., Gu, L., Li, L., et al.: Understanding the high activity of Fe–N–C electrocatalysts in oxygen reduction: fe/Fe3C nanoparticles boost the activity of Fe-Nx. J. Am. Chem. Soc. 138, 3570–3578 (2016)
Miao, Z., Wang, X., Tsai, M., et al.: Atomically dispersed Fe-Nx/C electrocatalyst boosts oxygen catalysis via a new metal-organic polymer supramolecule strategy. Adv Energy Mater 8(24), 1801226 (2018)
Qiao, B., Wang, A., Yang, X., et al.: Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011)
Yi, J., Xu, R., Wu, Q., et al.: Atomically dispersed iron–nitrogen active sites within porphyrinic triazine-based frameworks for oxygen reduction reaction in both alkaline and acidic media. ACS Energy Lett. 3, 883–889 (2018)
Long, J., Gang, W., Rui, Z., et al.: From metal-organic frameworks to single-atom Fe implanted N-doped porous carbons: efficient oxygen reduction in both alkaline and acidic media. Angew. Chem. Int. Ed. 130, 8661–8665 (2018)
Fei, H., Dong, J., Feng, Y., et al.: General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nat. Catal. 1, 63–72 (2018)
Zhang, H., Hwang, S., Wang, M., et al.: Single atomic iron catalysts for oxygen reduction in acidic media: particle size control and thermal activation. J. Am. Chem. Soc. 139, 14143–14149 (2017)
Chen, Y., Ji, S., Wang, Y., et al.: Isolated single iron atoms anchored on N-doped porous carbon as an efficient electrocatalyst for the oxygen reduction reaction. Angew. Chem. Int. Ed. 129, 7041–7045 (2017)
Choi, C.H., Choi, W.S., Kasian, O., et al.: Unraveling the nature of sites active toward hydrogen peroxide reduction in Fe–N–C catalysts. Angew. Chem. Int. Ed. 56, 8809–8812 (2017)
Herranz, J., Jaouen, F., Lefèvre, M., et al.: Unveiling N-protonation and anion-binding effects on Fe/N/C catalysts for O2 reduction in proton-exchange-membrane fuel cells. J. Phys. Chem. C 115, 16087–16097 (2011)
Kramm, U.I., Herranz, J., Larouche, N., et al.: Structure of the catalytic sites in Fe/N/C-catalysts for O2-reduction in PEM fuel cells. Phys. Chem. Chem. Phys. 14, 11673–11688 (2012)
Kramm, U.I., Herrmann-Geppert, I., Behrends, J., et al.: On an easy way to prepare metal-nitrogen doped carbon with exclusive presence of MeN4-type sites active for the ORR. J. Am. Chem. Soc. 138, 635–640 (2016)
Liu, J.J.: Advanced electron microscopy of metal-support interactions in supported metal catalysts. Chemcatchem 3, 934–948 (2011)
Gu, J., Cai, Z., Wang, D., et al.: Single-molecule imaging of iron-phthalocyanine-catalyzed oxygen reduction reaction by in situ scanning tunneling microscopy. ACS Nano 10, 8746–8750 (2016)
Zhong, W., Chen, J., Zhang, P., et al.: Air plasma etching towards rich active sites in Fe/N-porous carbon for the oxygen reduction reaction with superior catalytic performance. J. Mater. Chem. A 5, 16605–16610 (2017)
Tan, H., Li, Y., Jiang, X., et al.: Perfectly ordered mesoporous iron–itrogen doped carbon as highly efficient catalyst for oxygen reduction reaction in both alkaline and acidic electrolytes. Nano Energy 36, 286–294 (2017)
Ahn, S.H., Yu, X., Manthiram, A.: “Wiring” Fe-Nx-embedded porous carbon framework onto 1D nanotubes for efficient oxygen reduction reaction in alkaline and acidic media. Adv Mater 29(26), 1606534 (2017)
Chung, D.Y., Kim, M.J., Kang, N., et al.: Low-temperature and gram-scale synthesis of two-dimensional Fe–N–C carbon sheets for robust electrochemical oxygen reduction reaction. Chem. Mater. 29, 2890–2898 (2017)
Ding, Y., Niu, Y., Yang, J., et al.: A metal-amino acid complex-derived bifunctional oxygen electrocatalyst for rechargeable Zinc–air batteries. Small 12, 5414–5421 (2016)
Kozuch, S., Martin, J.M.L.: Turning over definitions in catalytic cycles. ACS Catal. 2, 2787–2794 (2012)
Cui, C., Gan, L., Heggen, M., et al.: Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis. Nat. Mater. 12, 765–771 (2013)
Li, M., Zhao, Z., Cheng, T., et al.: Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 354, 1414–1419 (2016)
Mahmood, J., Li, F., Jung, S.M., Okyay, M.S., et al.: An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction. Nat. Nanotech. 12, 441–446 (2017)
Malko, D., Kucernak, A.R.J., Lopes, T.: Performance of Fe–N/C oxygen reduction electrocatalysts towards NO2 −, NO, and NH2OH electroreduction-from fundamental insights into the active center to a new method for environmental nitrite destruction. J. Am. Chem. Soc. 138, 16056–16068 (2016)
Shen, H., Gracia-Espino, E., Ma, J., et al.: Synergistic effects between atomically dispersed Fe–N–C and C–S–C for the oxygen reduction reaction in acidic media. Angew. Chem. Int. Ed. 129, 13988–13992 (2017)
Varnell, J.A., Sotiropoulos, J.S., Brown, T.M., et al.: Revealing the role of the metal in non-precious-metal catalysts for oxygen reduction via selective removal of Fe. ACS Energy Lett. 3, 823–828 (2018)
Wu, G., More, K.L., Johnston, C.M., et al.: High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 332, 443–447 (2011)
Ye, Y., Li, H., Cai, F., et al.: Two-dimensional mesoporous carbon doped with Fe–N active sites for efficient oxygen reduction. ACS Catal. 7, 7638–7646 (2017)
Seh, Z.W., Kibsgaard, J., Dickens, C.F., et al.: Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, 146–157 (2017)
Wang, Y., Yuan, H., Li, Y., et al.: Two-dimensional iron-phthalocyanine (Fe–Pc) monolayer as a promising single-atom-catalyst for oxygen reduction reaction: a computational study. Nanoscale 7, 11633–11641 (2015)
Seo, M.H., Higgins, D., Jiang, G., et al.: Theoretical insight into highly durable iron phthalocyanine derived non-precious catalysts for oxygen reduction reactions. J. Mater. Chem. A 2, 19707–19716 (2014)
Titov, A., Zapol, P., Kral, P., et al.: Catalytic Fe-xN sites in carbon nanotubes. J. Phys. Chem. C 113, 21629–21634 (2009)
Liu, K., Wu, G., Wang, G.: Role of local carbon structure surrounding FeN4 Sites in boosting catalytic activity for oxygen reduction. J. Phys. Chem. C 121, 11319–11324 (2017)
Yasuda, S., Furuya, A., Uchibori, Y., et al.: Iron–nitrogen-doped vertically aligned carbon nanotube electrocatalyst for the oxygen reduction reaction. Adv. Funct. Mater. 26, 738–744 (2016)
Song, P., Wang, Y., Pan, J., et al.: Structure-activity relationship in high-performance iron-based electrocatalysts for oxygen reduction reaction. J. Power Sources 300, 279–284 (2015)
Jaouen, F., Marcotte, S., Dodelet, J.P., et al.: Oxygen reduction catalysts for polymer electrolyte fuel cells from the pyrolysis of iron acetate adsorbed on various carbon supports. J. Phys. Chem. B 107, 1376–1386 (2003)
Zhu, C., Shi, Q., Xu, B.Z., et al.: Hierarchically porous M–N–C (M = Co and Fe) single-atom electrocatalysts with robust MNx active moieties enable enhanced ORR performance. Adv Energy Mater 8(29), 1801956 (2018)
Hu, K., Tao, L., Liu, D., et al.: Sulfur-doped Fe/N/C nanosheets as highly efficient electrocatalysts for oxygen reduction reaction. ACS Appl. Mater. Interfaces 8, 19379–19385 (2016)
Kone, I., Xie, A., Tang, Y., et al.: Hierarchical porous carbon doped with iron–nitrogen–sulfur for efficient oxygen reduction reaction. ACS Appl. Mater. Interfaces 9, 20963–20973 (2017)
Men, B., Sun, Y., Liu, J., et al.: Synergistically enhanced electrocatalytic activity of sandwich-like N-doped graphene/carbon nanosheets decorated by Fe and S for oxygen reduction reaction. ACS Appl. Mater. Interfaces 8, 19533–19541 (2016)
Sasan, K., Kong, A., Wang, Y., et al.: From hemoglobin to porous N–S–Fe–doped carbon for efficient oxygen electroreduction. J. Phys. Chem. C 119, 13545–13550 (2015)
Kwak, D.H., Han, S.B., Lee, Y.W., et al.: Fe/N/S-doped mesoporous carbon nanostructures as electrocatalysts for oxygen reduction reaction in acid medium. Appl. Catal. B Environ. 203, 889–898 (2017)
Wang, Y.C., Lai, Y.J., Song, L., et al.: S-doping of an Fe/N/C ORR catalyst for polymer electrolyte membrane fuel cells with high power density. Angew. Chem. Int. Ed. 127, 10045–10048 (2015)
Sun, M., Davenport, D., Liu, H., et al.: Highly efficient and sustainable non-precious-metal Fe–N–C electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 6, 2527–2539 (2018)
Zhang, J., Byeon, A., Lee, J.W.: Boron-doped carbon-iron nanocomposites as efficient oxygen reduction electrocatalysts derived from carbon dioxide. Chem. Commun. 50, 6349–6352 (2014)
Yuan, K., Sfaelou, S., Qiu, M., et al.: Synergetic contribution of boron and Fe-N-x species in porous carbons toward efficient electrocatalysts for oxygen reduction reaction. Acs Energy Lett. 3, 252–260 (2018)
Fajrial, A.K., Saputro, A.G., Agusta, M.K., et al.: First principles study of oxygen molecule interaction with the graphitic active sites of a boron-doped pyrolyzed Fe–N–C catalyst. Phy. Chem. Chem. Phy. 19, 23497–23504 (2017)
Chen, P., Zhou, T., Xing, L., et al.: Atomically dispersed iron-nitrogen species as electrocatalysts for bifunctional oxygen evolution and reduction reactions. Angew. Chem. Int. Ed. 56, 610–614 (2017)
Kramm, U.I., Herrmann-Geppert, I., Fiechter, S., et al.: Effect of iron-carbide formation on the number of active sites in Fe–N–C catalysts for the oxygen reduction reaction in acidic media. J. Mater. Chem. A 2, 2663–2670 (2014)
He, Z., Maurice, J.L., Gohier, A., et al.: Iron catalysts for the growth of carbon nanofibers: Fe, Fe3C or both? Chem. Mater. 23, 5379–5387 (2011)
Fan, X., Peng, Z., Ye, R., et al.: M3C (M: Fe Co, Ni) nanocrystals encased in graphene nanoribbons: an active and stable bifunctional electrocatalyst for oxygen reduction and hydrogen evolution reactions. ACS Nano 9, 7407–7418 (2015)
Hou, Y., Huang, T., Wen, Z., et al.: Metal-organic framework-derived nitrogen-doped core-shell-structured porous Fe/Fe3C@C nanoboxes supported on graphene sheets for efficient oxygen reduction reactions. Adv. Energy Mater. 4, 1220–1225 (2014)
Hu, Y., Jensen, J.O., Zhang, W., et al.: Hollow spheres of iron carbide nanoparticles encased in graphitic layers as oxygen reduction catalysts. Angew. Chem. Int. Ed. 53, 3675–3679 (2014)
Zhang, Z., Sun, J., Wang, F., et al.: Efficient oxygen reduction reaction (ORR) catalysts based on single iron atoms dispersed on a hierarchically structured porous carbon framework. Angew. Chem. Int. Ed. 130, 9176–9181 (2018)
Han, S., Hu, X., Wang, J., et al.: Novel route to Fe-based cathode as an efficient bifunctional catalysts for rechargeable Zn–air battery. Adv. Energy Mater. 8, 1800955 (2018)
Ding, W., Li, L., Xiong, K., et al.: Shape fixing via salt recrystallization: a morphology-controlled approach to convert nanostructured polymer to carbon nanomaterial as a highly active catalyst for oxygen reduction reaction. J. Am. Chem. Soc. 137, 5414–5420 (2015)
Yang, L., Cheng, D., Xu, H., et al.: Unveiling the high-activity origin of single-atom iron catalysts for oxygen reduction reaction. PNAS 115, 6626–6631 (2018)
Serov, A., Artyushkova, K., Atanassov, P.: Fe–N–C oxygen reduction fuel cell catalyst derived from carbendazim: synthesis, structure, and reactivity. Adv. Energy Mater. 4, 1301735 (2014)
Lefevre, M., Proietti, E., Jaouen, F., et al.: Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science 324, 71–74 (2009)
Kim, J., Lee, J., Choi, Y., et al.: Synthesis of hierarchical linearly assembled graphitic carbon nanoparticles via catalytic graphitization in SBA-15. Carbon 75, 95–103 (2014)
Jiao, Y., Han, D., Liu, L., et al.: Highly ordered mesoporous few-layer graphene frameworks enabled by Fe3O4 nanocrystal superlattices. Angew. Chem. Int. Ed. 54, 5727–5731 (2015)
Wu, Z., Xu, X., Hu, B., et al.: Iron carbide nanoparticles encapsulated in mesoporous Fe–N-doped carbon nanofibers for efficient electrocatalysis. Angew. Chem. Int. Ed. 54, 8179–8183 (2015)
Liu, D., Long, Y.: Superior catalytic activity of electrochemically reduced graphene oxide supported iron phthalocyanines toward oxygen reduction reaction. ACS Appl. Mater. Interfaces 7, 24063–24068 (2015)
Zhou, T., Zhou, Y., Ma, R., et al.: Achieving excellent activity and stability for oxygen reduction electrocatalysis by hollow mesoporous iron-nitrogen-doped graphitic carbon spheres. J. Mater. Chem. A 5, 12243–12251 (2017)
Liang, H.W., Wei, W., Wu, Z.-S., et al.: Mesoporous metal-nitrogen-doped carbon electrocatalysts for highly efficient oxygen reduction reaction. J. Am. Chem. Soc. 135, 16002–16005 (2013)
Kong, A., Dong, B., Zhu, X., et al.: Ordered mesoporous Fe-porphyrin-like architectures as excellent cathode materials for the oxygen reduction reaction in both alkaline and acidic media. Chemistry 19, 16170–16175 (2013)
Song, L., Wu, Z., Zhou, F., et al.: Sustainable hydrothermal carbonization synthesis of iron/nitrogen-doped carbon nanofiber aerogels as electrocatalysts for oxygen reduction. Small 12, 6398–6406 (2016)
Wang, B., Wang, X., Zou, J., et al.: Simple-cubic carbon frameworks with atomically dispersed iron dopants toward high-efficiency oxygen reduction. Nano Lett. 17, 2003–2009 (2017)
Ma, R., Zhou, Y., Hu, C., et al.: Post iron-doping of activated nitrogen-doped carbon spheres as a high-activity oxygen reduction electrocatalyst. Energy Storage Mater. 13, 142–150 (2018)
Deng, Y., Dong, Y., Wang, G., et al.: Well-defined ZIF-derived Fe–N co-doped carbon nanoframes as efficient oxygen reduction catalysts. ACS Appl. Mater. Interfaces 9, 9699–9709 (2017)
Liu, T., Li, M., Jiao, C., et al.: Design and synthesis of integrally structured Ni3N nanosheets/carbon microfibers/Ni3 N nanosheets for efficient full water splitting catalysis. J. Mater. Chem. A 5, 9377–9390 (2017)
Li, J., Chen, S., Li, W., et al.: A eutectic salt-assisted semi-closed pyrolysis route to fabricate high-density active-site hierarchically porous Fe/N/C catalysts for the oxygen reduction reaction. J. Mater. Chem. A 6, 15504–15509 (2018)
Gewirth, A.A., Varnell, J.A., DiAscro, A.M.: Nonprecious metal catalysts for oxygen reduction in heterogeneous aqueous systems. Chem. Rev. 118, 2313–2339 (2018)
Wang, X.X., Cullen, D.A., Pan, Y.T., et al.: Nitrogen-coordinated single cobalt atom catalysts for oxygen reduction in proton exchange membrane fuel cells. Adv. Mater. 30, 1706758 (2018)
Zitolo, A., Ranjbar-Sahraie, N., Mineva, T., et al.: Identification of catalytic sites in cobalt-nitrogen-carbon materials for the oxygen reduction reaction. Nat. Commun. 8, 957 (2017)
Yin, P., Yao, T., Wu, Y., et al.: Single cobalt atoms with precise n-coordination as superior oxygen reduction reaction catalysts. Angew. Chem. Int. Ed. 55, 10800–10805 (2016)
Hu, Z., Guo, Z., Zhang, Z., et al.: Bimetal zeolitic imidazolite framework-derived iron-, cobalt- and nitrogen-codoped carbon nanopolyhedra electrocatalyst for efficient oxygen reduction. ACS Appl. Mater. Interfaces 10, 12651–12658 (2018)
Guan, Z., Zhang, X., Chen, W., et al.: Mesoporous S doped Fe–N–C materials as highly active oxygen reduction reaction catalyst. Chem. Commun. (2018). https://doi.org/10.1039/c8cc05273e
Guo, D., Shibuya, R., Akiba, C., et al.: Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 351, 361–365 (2016)
Liang, J., Zhou, R.F., Chen, X.M., et al.: Fe-N decorated hybrids of CNTs grown on hierarchically porous carbon for high-performance oxygen reduction. Adv. Mater. 26, 6074–6079 (2014)
Yang, Z.X., Xia, Y.D., Mokaya, R.: Aligned N-doped carbon nanotube bundles prepared via CVD using zeolite substrates. Chem. Mater. 17, 4502–4508 (2005)
Sheng, Z.H., Shao, L., Chen, J.J., et al.: Catalyst-free synthesis of nitrogen-doped graphene via thermal annealing graphite oxide with melamine and its excellent electrocatalysis. ACS Nano 5, 4350–4358 (2011)
Kundu, S., Nagaiah, T.C., Xia, W., et al.: Electrocatalytic activity and stability of nitrogen-containing carbon nanotubes in the oxygen reduction reaction. J. Phys. Chem. C 113, 14302–14310 (2009)
Nagaiah, T.C., Kundu, S., Bron, M., et al.: Nitrogen-doped carbon nanotubes as a cathode catalyst for the oxygen reduction reaction in alkaline medium. Electrochem. Commun. 12, 338–341 (2010)
Liu, R., Wu, D., Feng, X., et al.: Nitrogen-doped ordered mesoporous graphitic arrays with high electrocatalytic activity for oxygen reduction. Angew. Chem. Int. Ed. 49, 2565–2569 (2010)
Matter, P., Wang, E., Ozkan, U.: Preparation of nanostructured nitrogen-containing carbon catalysts for the oxygen reduction reaction from SiO2- and MgO-supported metal particles. J. Catal. 243, 395–403 (2006)
Mo, Z., Liao, S., Zheng, Y., et al.: Preparation of nitrogen-doped carbon nanotube arrays and their catalysis towards cathodic oxygen reduction in acidic and alkaline media. Carbon 50, 2620–2627 (2012)
Webster, S., Maultzsch, J., Thomsen, C., et al.: Raman characterization of nitrogen doped multiwalled carbon nanotubes. Nanotub. Devices 772, 129–134 (2003)
Van Dommele, S., Romero-Izquirdo, A., Brydson, R., et al.: Tuning nitrogen functionalities in catalytically grown nitrogen-containing carbon nanotubes. Carbon 46, 138–148 (2008)
Lin, L., Yang, Z.K., Jiang, F., et al.: Nonprecious bimetallic (Fe, Mo)-N/C catalyst for efficient oxygen reduction reaction. ACS Catal. 6, 4449–4454 (2016)
Wang, J., Qiao, J., Baker, R., et al.: Alkaline polymer electrolyte membranes for fuel cell applications. Chem. Soc. Rev. 42, 5768–5787 (2013)
Zhou, T., Shao, R., Chen, S., et al.: A review of radiation-grafted polymer electrolyte membranes for alkaline polymer electrolyte membrane fuel cells. J. Power Sources 293, 946–975 (2015)
O’Hayre, R., Cha, S.W., Colella, W., et al.: Fuel Cell Fundamentals. Wiley, New York (2016)
Spendelow, J.S., Papageorgopoulos, D.C.: Progress in PEMFC MEA component R&D at the DOE fuel cell technologies program. Fuel Cells 11, 775–786 (2011)
Gasteiger, H.A., Kocha, S.S., Sompalli, B., et al.: Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B Environ. 56, 9–35 (2005)
Milliken, J. Hydrogen, fuel cells and infrastructure technologies program, multi-year research, development and demonstration plan. US Department of Energy, Page 3.4–2 (2007). https://doi.org/10.2172/920934
Liu, Q., Liu, X., Zheng, L., et al.: The solid-phase synthesis of an Fe–N–C electrocatalyst for high-power proton-exchange membrane fuel cells. Angew. Chem. Int. Ed. 57, 1204–1208 (2018)
Proietti, E., Jaouen, F., Lefevre, M., et al.: Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells. Nat. Commun. 2, 416 (2011)
Yuan, S., Shui, L., Grabstanowicz, L., et al.: A highly active and support-free oxygen reduction catalyst prepared from ultrahigh-surface-area porous polyporphyrin. Angew. Chem. Int. Ed. 125, 8507–8511 (2013)
Wang, J., Huang, Z., Liu, W., et al.: Design of N-coordinated dual-metal sites: a stable and active Pt-free catalyst for acidic oxygen reduction reaction. J. Am. Chem. Soc. 139, 17281–17284 (2017)
Fu, X., Zamani, P., Choi, J.Y., et al.: In situ polymer graphenization ingrained with nanoporosity in a nitrogenous electrocatalyst boosting the performance of polymer-electrolyte-membrane fuel cells. Adv. Mater. 29, 1604456 (2017)
Wu, Y., Wang, C., Wang, R., et al.: Three-dimensional networks of S-doped Fe/N/C with hierarchical porosity for efficient oxygen reduction in polymer electrolyte membrane fuel cells. ACS Appl. Mater. Interfaces 10, 14602–14613 (2018)
Meng, F., Liu, K., Zhang, Y., et al.: Recent advances toward the rational design of efficient bifunctional air electrodes for rechargeable Zn–air batteries. Small 14, 1703843 (2018)
Ganesan, P., Prabu, M., Sanetuntikul, J., et al.: Cobalt sulfide nanoparticles grown on nitrogen and sulfur codoped graphene oxide: an efficient electrocatalyst for oxygen reduction and evolution reactions. ACS Catal. 5, 3625–3637 (2015)
Shinde, S.S., Lee, C.H., Sami, A., et al.: Scalable 3-D carbon nitride sponge as an efficient metal-free bifunctional oxygen electrocatalyst for rechargeable Zn–air batteries. ACS Nano 11, 347–357 (2017)
Wang, R., Chen, Z., Hu, N., et al.: Nanocarbon-based electrocatalysts for rechargeable aqueous Li/Zn–air batteries. ChemElectroChem 5, 1745–1763 (2018)
Pan, J., Xu, Y.Y., Yang, H., et al.: Advanced architectures and relatives of air electrodes in Zn–air batteries. Adv. Sci. 5, 1700691 (2018)
Ma, L., Yu, T., Tzoganakis, E., et al.: Fundamental understanding and material challenges in rechargeable nonaqueous Li–O2 batteries: recent progress and perspective. Adv. Energy Mater. 8, 1800348 (2018)
Huang, Y., Wang, Y., Tang, C., et al.: Atomic modulation and structure design. Adv. Mater. (2018). https://doi.org/10.1002/adma.201803800
Li, Z., Yang, J., Xu, G., et al.: Non-precious cathode electrocatalyst for magnesium air fuel cells: activity and durability of iron-polyphthalocyanine absorbed on carbon black. J. Power Sources 242, 157–165 (2013)
Shui, J., Karan, N.K., Balasubramanian, M., et al.: Fe/N/C composite in Li–O2 battery: studies of catalytic structure and activity toward oxygen evolution reaction. J. Am. Chem. Soc. 134, 16654–16661 (2012)
Yu, L., Shen, Y., Huang, Y.: Fe–N–C catalyst modified graphene sponge as a cathode material for lithium–oxygen battery. J. Alloys. Compd. 595, 185–191 (2014)
Wei, W., Shi, X., Gao, P., et al.: Well-elaborated, mechanochemically synthesized Fe-TPP subset of ZIF precursors (Fe-TPP = tetraphenylporphine iron) to atomically dispersed iron-nitrogen species for oxygen reduction reaction and Zn–air batteries. Nano Energy 52, 29–37 (2018)
Wei, Q., Zhang, G., Yang, X., et al.: Litchi-like porous Fe/N/C spheres with atomically dispersed FeNx promoted by sulfur as highly efficient oxygen electrocatalysts for Zn–air batteries. J. Mater. Chem. A 6, 4605–4610 (2018)
Pan, Y., Liu, S., Sun, K., et al.: A bimetallic Zn/Fe polyphthalocyanine-derived single-atom Fe-N4 catalytic site: a superior trifunctional catalyst for overall water splitting and Zn–air batteries. Angew. Chem. Int. Ed. 57, 8614–8618 (2018)
Jin, S.: Are metal chalcogenides, nitrides, and phosphides oxygen evolution catalysts or bifunctional catalysts? ACS Energy Lett. 2, 1937–1938 (2017)
Hu, B., Wu, Z., Chu, S., et al.: SiO2-protected shell mediated templating synthesis of Fe–N-doped carbon nanofibers and their enhanced oxygen reduction reaction performance. Energy Environ. Sci. 11, 2208–2215 (2018)
Tian, N., Lu, B., Yang, X., et al.: Rational design and synthesis of low-temperature fuel cell electrocatalysts. Electrochem. Energy Rev. 1, 54–83 (2018)
Chenitz, R., Kramm, U.I., Lefevre, M., et al.: A specific demetalation of Fe-N4 catalytic sites in the micropores of NC_Ar + NH3 is at the origin of the initial activity loss of the highly active Fe/N/C catalyst used for the reduction of oxygen in PEM fuel cells. Energy Environ. Sci. 11, 365–382 (2018)
Acknowledgements
This work is supported by the National Natural Science Foundation of China (21471147) and the National Key Research and Development Program of China (2016YFB0101200). M. Yang appreciates the support from the Ningbo 3315 program. J. Wang would like to thank the financial support from the Science and Technology Commission of Shanghai Municipality (15520720400, 16DZ2260603) and the Equipment Research Program (6140721050215). Tiju Thomas would like to thank the Indian Institute of Technology, Madras and the Department of Science and Technology (DST, Government of India) for their financial support in the form of grants such as the Young Scientist Scheme (YSS/2015/001712) and the Centre Grant CHY1718383DSTXTPRA and SOL1819001DSTXHOCX. Tiju Thomas would also like to thank the Ministry of Electronics & Information Technology for their support and the Centre grant ELE1819353MEITENAK (in which he is a Center member). H. Shen would like to acknowledge the contributions made by Liu Yang at the Beijing Advanced Innovation Center for Soft Matter Science and Engineering at the Beijing University of Chemical Technology.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Shen, H., Thomas, T., Rasaki, S.A. et al. Oxygen Reduction Reactions of Fe-N-C Catalysts: Current Status and the Way Forward. Electrochem. Energ. Rev. 2, 252–276 (2019). https://doi.org/10.1007/s41918-019-00030-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41918-019-00030-w