Abstract
Rechargeable potassium-ion batteries (KIBs) are potential alternatives to lithium-ion batteries for application in large-scale energy storage systems due to their inexpensive and highly abundant resources. Recently, various anode materials have been investigated for use in KIBs, especially the traditional graphite anodes which have already been successfully applied in KIBs. In contrast, the appropriate cathode materials which are able to accommodate large K ions are urgently needed. In this review, a comprehensive summary of the latest advancements in cathode materials for non-aqueous KIBs in terms of capacity, cycle life and energy density will be presented, as well as K-storage mechanisms. In addition, various strategies to improve K-storage performance will be provided through combining insights from the study of material structures and properties and thus bring low-cost non-aqueous KIBs a step closer to application in sustainable large-scale energy storage systems.
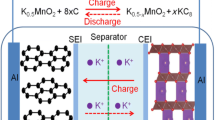
Graphical Abstract















Similar content being viewed by others
References
Armand, M., Tarascon, J.M.: Building better batteries. Nature 451, 652–657 (2008)
Dunn, B., Kamath, H., Tarascon, J.M.: Electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011)
Hwang, J.Y., Myung, S.T., Sun, Y.K.: Sodium-ion batteries: present and future. Chem. Soc. Rev. 46, 3529–3614 (2017)
Kundu, D., Talaie, E., Duffort, V., et al.: The emerging chemistry of sodium ion batteries for electrochemical energy storage. Angew. Chem. Int. Edit. 54, 3431–3448 (2015)
Yang, C.J., Jackson, R.B.: Opportunities and barriers to pumped-hydro energy storage in the United States. Renew. Sustain. Energy Rev. 15, 839–844 (2011)
Saidur, R., Rahim, N.A., Hasanuzzaman, M.: A review on compressed-air energy use and energy savings. Renew. Sustain. Energy Rev. 14, 1135–1153 (2010)
Bolund, B., Bernhoff, H., Leijon, M.: Flywheel energy and power storage systems. Renew. Sustain. Energy Rev. 11, 235–258 (2007)
Yang, Z., Zhang, J., Kintner-Meyer, M.C.W., et al.: Electrochemical energy storage for green grid. Chem. Rev. 111, 3577–3613 (2011)
Häupler, B., Wild, A., Schubert, U.S.: Carbonyls: powerful organic materials for secondary batteries. Adv. Energy Mater. 5, 1402034 (2015)
Muldoon, J., Bucur, C.B., Gregory, T.: Quest for nonaqueous multivalent secondary batteries: magnesium and beyond. Chem. Rev. 114, 11683–11720 (2014)
Li, Z., Huang, J., Liaw, B.Y., et al.: A review of lithium deposition in lithium-ion and lithium metal secondary batteries. J. Power Sources 254, 168–182 (2014)
Xu, C., Chen, Y., Shi, S., et al.: Secondary batteries with multivalent ions for energy storage. Sci. Rep. 5, 14120 (2015)
Tarascon, J.M., Armand, M.: Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001)
Choi, J.W., Aurbach, D.: Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 1, 16013 (2016)
Croguennec, L., Palacin, M.R.: Recent achievements on inorganic electrode materials for lithium-ion batteries. J. Am. Chem. Soc. 137, 3140–3156 (2015)
Slater, M.D., Kim, D., Lee, E., et al.: Sodium-ion batteries. Adv. Funct. Mater. 23, 947–958 (2013)
Speirs, J., Contestabile, M., Houari, Y., et al.: The future of lithium availability for electric vehicle batteries. Renew. Sustain. Energy Rev. 35, 183–193 (2014)
Olivetti, E.A., Ceder, G., Gaustad, G.G., et al.: Lithium-ion battery supply chain considerations: analysis of potential bottlenecks in critical metals. Joule 1, 229–243 (2017)
Pan, H., Hu, Y.S., Chen, L.: Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 6, 2338–2360 (2013)
Kim, H., Kim, J.C., Bianchini, M., et al.: Recent progress and perspective in electrode materials for K-ion batteries. Adv. Energy Mater. 8, 1702384 (2018)
Aurbach, D., Lu, Z., Schechter, A., et al.: Prototype systems for rechargeable magnesium batteries. Nature 407, 724–727 (2000)
Wang, M., Jiang, C., Zhang, S., et al.: Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage. Nat. Chem. 10, 667–672 (2018)
Lin, M.C., Gong, M., Lu, B., et al.: An ultrafast rechargeable aluminium-ion battery. Nature 520, 325–328 (2015)
Okoshi, M., Yamada, Y., Komaba, S., et al.: Theoretical analysis of interactions between potassium ions and organic electrolyte solvents: a comparison with lithium, sodium, and magnesium ions. J. Electrochem. Soc. 164, 54–60 (2017)
Lei, K., Li, F., Mu, C., et al.: High K-storage performance based on the synergy of dipotassium terephthalate and ether-based electrolytes. Energy Environ. Sci. 10, 552–557 (2017)
Wu, X., Leonard, D.P., Ji, X.: Emerging non-aqueous potassium-ion batteries: challenges and opportunities. Chem. Mater. 29, 5031–5042 (2017)
Pramudita, J.C., Sehrawat, D., Goonetilleke, D., et al.: An initial review of the status of electrode materials for potassium-ion batteries. Adv. Energy Mater. 7, 1602911 (2017)
Sultana, I., Rahman, M.M., Chen, Y., et al.: Potassium-ion battery anode materials operating through the alloying–dealloying reaction mechanism. Adv. Funct. Mater. 28, 1703857 (2018)
Komaba, S., Hasegawa, T., Dahbi, M., et al.: Potassium intercalation into graphite to realize high-voltage/high-power potassium-ion batteries and potassium-ion capacitors. Electrochem. Commun. 60, 172–175 (2015)
Jian, Z., Luo, W., Ji, X.: Carbon electrodes for K-ion batteries. J. Am. Chem. Soc. 137, 11566–11569 (2015)
McCulloch, W.D., Ren, X., Yu, M., et al.: Potassium-ion oxygen battery based on a high capacity antimony anode. ACS Appl. Mater. Interfaces 7, 26158–26166 (2015)
Zhang, W., Mao, J., Li, S., et al.: Phosphorus-based alloy materials for advanced potassium-ion battery anode. J. Am. Chem. Soc. 139, 3316–3319 (2017)
Gao, H., Zhou, T., Zheng, Y., et al.: CoS quantum dot nanoclusters for high-energy potassium-ion batteries. Adv. Funct. Mater. 27, 1702634 (2017)
Xie, K., Yuan, K., Li, X., et al.: Superior potassium ion storage via vertical MoS2 “nano-rose” with expanded interlayers on graphene. Small 13, 1701471 (2017)
Deng, Q., Pei, J., Fan, C., et al.: Potassium salts of para-aromatic dicarboxylates as the highly efficient organic anodes for low-cost K-ion batteries. Nano Energy 33, 350–355 (2017)
Qian, J., Wu, C., Cao, Y., et al.: Prussian blue cathode materials for sodium-ion batteries and other ion batteries. Adv. Energy Mater. 8, 1702619 (2018)
Zhu, Y., Yuan, S., Bao, D., et al.: Decorating waste cloth via industrial wastewater for tube-type flexible and wearable sodium-ion batteries. Adv. Mater. 29, 1603719 (2017)
Mizuno, Y., Okubo, M., Hosono, E., et al.: Electrochemical Mg2+ intercalation into a bimetallic CuFe Prussian blue analog in aqueous electrolytes. J. Mater. Chem. A 1, 13055–13059 (2013)
Lipson, A.L., Pan, B., Lapidus, S.H., et al.: Rechargeable Ca-ion batteries: a new energy storage system. Chem. Mater. 27, 8442–8447 (2015)
Eftekhari, A.: Potassium secondary cell based on Prussian blue cathode. J. Power Sources 126, 221–228 (2004)
Zhang, C., Xu, Y., Zhou, M., et al.: Potassium Prussian blue nanoparticles: a low-cost cathode material for potassium-ion batteries. Adv. Funct. Mater. 27, 1604307 (2017)
Chong, S., Chen, Y., Zheng, Y., et al.: Potassium ferrous ferricyanide nanoparticles as a high capacity and ultralong life cathode material for nonaqueous potassium-ion batteries. J. Mater. Chem. A 5, 22465–22471 (2017)
Shadike, Z., Shi, D.R., Cao, M.H., et al.: Long life and high-rate Berlin green FeFe(CN)6 cathode material for a non-aqueous potassium-ion battery. J. Mater. Chem. A 5, 6393–6398 (2017)
He, G., Nazar, L.F.: Crystallite size control of Prussian White analogues for nonaqueous potassium-ion batteries. ACS Energy Lett. 2, 1122–1127 (2017)
Liao, J., Hu, Q., Yu, Y., et al.: A potassium-rich iron hexacyanoferrate/dipotassium terephthalate@carbon nanotube composite used for K-ion full-cells with an optimized electrolyte. J. Mater. Chem. A 5, 19017–19024 (2017)
Xue, L., Li, Y., Gao, H., et al.: Low-cost high-energy potassium cathode. J. Am. Chem. Soc. 139, 2164–2167 (2017)
Bie, X., Kubota, K., Hosaka, T., et al.: A novel K-ion battery: hexacyanoferrate(II)/graphite cell. J. Mater. Chem. A 5, 4325–4330 (2017)
Song, J., Wang, L., Lu, Y., et al.: Removal of interstitial H2O in hexacyanometallates for a superior cathode of a sodium-ion battery. J. Am. Chem. Soc. 137, 2658–2664 (2015)
Wu, X., Jian, Z., Li, Z., et al.: Prussian white analogues as promising cathode for non-aqueous potassium-ion batteries. Electrochem. Commun. 77, 54–57 (2017)
Ren, W., Qin, M., Zhu, Z., et al.: Activation of sodium storage sites in Prussian blue analogues via surface etching. Nano Lett. 17, 4713–4718 (2017)
Poizot, P., Laruelle, S., Grugeon, S., et al.: Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 407, 496–499 (2000)
Wang, Y., Yu, X., Xu, S., et al.: A zero-strain layered metal oxide as the negative electrode for long-life sodium-ion batteries. Nat. Commun. 4, 2365 (2013)
Xu, B., Fell, C.R., Chi, M., et al.: Identifying surface structural changes in layered Li-excess nickel manganese oxides in high voltage lithium ion batteries: a joint experimental and theoretical study. Energy Environ. Sci. 4, 2223–2233 (2011)
Yabuuchi, N., Kajiyama, M., Iwatate, J., et al.: P2-type Nax[Fe1/2Mn1/2]O2 made from earth-abundant elements for rechargeable Na batteries. Nat. Mater. 11, 512–517 (2012)
Vaalma, C., Giffin, G.A., Buchholz, D., et al.: Non-aqueous K-ion battery based on layered K0.3MnO2 and hard carbon/carbon black. J. Electrochem. Soc. 163, 1295–1299 (2016)
Hironaka, Y., Kubota, K., Komaba, S.: P2-and P3-KxCoO2 as an electrochemical potassium intercalation host. Chem. Commun. 53, 3693–3696 (2017)
Kim, H., Kim, J.C., Bo, S.H., et al.: K-ion batteries based on a P2-type K0.6CoO2 cathode. Adv. Energy Mater. 7, 1700098 (2017)
Jiang, J., Li, Y., Liu, J., et al.: Recent advances in metal oxide-based electrode architecture design for electrochemical energy storage. Adv. Mater. 24, 5166–5180 (2012)
Hwang, J.Y., Oh, S.M., Myung, S.T., et al.: Radially aligned hierarchical columnar structure as a cathode material for high energy density sodium-ion batteries. Nat. Commun. 6, 6865 (2015)
Deng, T., Fan, X., Luo, C., et al.: Self-templated formation of P2-type K0.6CoO2 microspheres for high reversible potassium-ion batteries. Nano Lett. 18, 1522–1529 (2018)
Liu, C., Luo, S., Huang, H., et al.: K0.67Ni0.17Co0.17Mn0.66O2: a cathode material for potassium-ion battery. Electrochem. Commun. 82, 150–154 (2017)
Kim, H., Seo, D.H., Kim, J.C., et al.: Investigation of potassium storage in layered P3-type K0.5MnO2 cathode. Adv. Mater. 29, 1702480 (2017)
Gong, Z., Yang, Y.: Recent advances in the research of polyanion-type cathode materials for Li-ion batteries. Energy Environ. Sci. 4, 3223–3242 (2011)
Barpanda, P., Ati, M., Melot, B.C., et al.: A 3.90 V iron-based fluorosulphate material for lithium-ion batteries crystallizing in the triplite structure. Nat. Mater. 10, 772–779 (2011)
Masquelier, C., Croguennec, L.: Polyanionic (phosphates, silicates, sulfates) frameworks as electrode materials for rechargeable Li (or Na) batteries. Chem. Rev. 113, 6552–6591 (2013)
Barpanda, P., Oyama, G., Nishimura, S., et al.: A 3.8-V earth-abundant sodium battery electrode. Nat. Commun. 5, 4358 (2014)
Recham, N., Rousse, G., Sougrati, M.T., et al.: Preparation and characterization of a stable FeSO4F-based framework for alkali ion insertion electrodes. Chem. Mater. 24, 4363–4370 (2012)
Mathew, V., Kim, S., Kang, J., et al.: Amorphous iron phosphate: potential host for various charge carrier ions. NPG Asia Mater. 6, 138 (2014)
Han, J., Li, G.N., Liu, F., et al.: Investigation of K3V2(PO4)3/C nanocomposites as high-potential cathode materials for potassium-ion batteries. Chem. Commun. 53, 1805–1808 (2017)
Chihara, K., Katogi, A., Kubota, K., et al.: KVPO4F and KVOPO4 toward 4 volt-class potassium-ion batteries. Chem. Commun. 53, 5208–5211 (2017)
Song, W., Cao, X., Wu, Z., et al.: Investigation of the sodium ion pathway and cathode behavior in Na3V2(PO4)2F3 combined via a first principles calculation. Langmuir 30, 12438–12446 (2014)
Lin, X., Huang, J., Tan, H., et al.: K3V2(PO4)2F3 as a robust cathode for potassium-ion batteries. Energy Storage Mater. 16, 97–101 (2018)
Park, W.B., Han, S.C., Park, C., et al.: KVP2O7 as a robust high-energy cathode for potassium-ion batteries: pinpointed by a full screening of the inorganic registry under specific search conditions. Adv. Energy Mater. 8, 1703099 (2017)
Liang, Y., Tao, Z., Chen, J., et al.: Organic electrode materials for rechargeable lithium batteries. Adv. Energy Mater. 2, 742–769 (2012)
Oyama, N., Tatsuma, T., Sotomura, T.: Organosulfur polymer batteries with high energy density. J. Power Sources 68, 135–138 (1997)
Yao, M., Senoh, H., Yamazaki, S., et al.: High-capacity organic positive-electrode material based on a benzoquinone derivative for use in rechargeable lithium batteries. J. Power Sources 195, 8336–8340 (2010)
Liang, Y., Zhang, P., Yang, S., et al.: Fused heteroaromatic organic compounds for high-power electrodes of rechargeable lithium batteries. Adv. Energy Mater. 3, 600–605 (2013)
Han, X., Chang, C., Yuan, L., et al.: Aromatic carbonyl derivative polymers as high-performance Li-ion storage materials. Adv. Mater. 19, 1616–1621 (2007)
Luo, W., Allen, M., Raju, V., et al.: An organic pigment as a high-performance Cathode for sodium-ion batteries. Adv. Energy Mater. 4, 1400554 (2014)
Wang, H., Yuan, S., Si, Z., et al.: Multi-ring aromatic carbonyl compounds enabling high capacity and stable performance of sodium-organic batteries. Energy Environ. Sci. 8, 3160–3165 (2015)
Zhu, Y., Yang, X., Zhang, X.: Hydronium ion batteries: a sustainable energy storage solution. Angew. Chem. Int. Ed. 56, 6378–6380 (2017)
Chen, Y., Luo, W., Carter, M., et al.: Organic electrode for non-aqueous potassium-ion batteries. Nano Energy 18, 205–211 (2015)
Song, Z.P., Zhan, H., Zhou, Y.H.: Anthraquinone based polymer as high performance cathode material for rechargeable lithium batteries. Chem. Commun. 4, 448–450 (2009)
Zhang, K., Guo, C.Y., Zhao, Q., et al.: High-performance organic lithium batteries with an ether-based electrolyte and 9,10-anthraquinone (AQ)/CMK-3 cathode. Adv. Sci. 2, 1500018 (2015)
Zhang, Y., Huang, Y.S., Yang, G.H., et al.: Dispersion–assembly approach to synthesize three-dimensional graphene/polymer composite aerogel as a powerful organic cathode for rechargeable Li and Na batteries. ACS Appl. Mater. Interfaces 9, 15549–15556 (2017)
Wu, Y.W., Zeng, R.H., Nan, J.M., et al.: Quinone electrode materials for rechargeable lithium/sodium ion batteries. Adv. Energy Mater. 7, 1700278 (2017)
Song, Z.P., Qian, Y.M., Gordin, M.L., et al.: Polyanthraquinone as a reliable organic electrode for stable and fast lithium storage. Angew. Chem. Int. Ed. 54, 13947–13951 (2015)
Wan, W., Lee, H.S., Yu, X.Q., et al.: Tuning the electrochemical performances of anthraquinone organic cathode materials for Li-ion batteries through the sulfonic sodium functional group. RSC Adv. 4, 19878–19882 (2014)
Jian, Z., Liang, Y., Rodríguez-Pérez, I.A., et al.: Poly (anthraquinonyl sulfide) cathode for potassium-ion batteries. Electrochem. Commun. 71, 5–8 (2016)
Zhao, J., Yang, J., Sun, P., et al.: Sodium sulfonate groups substituted anthraquinone as an organic cathode for potassium batteries. Electrochem. Commun. 86, 34–37 (2018)
Zhu, Y.H., Yang, X., Bao, D., et al.: High-energy-density flexible potassium-ion battery based on patterned electrodes. Joule 2, 736–746 (2018)
Jian, Z., Xing, Z., Bommier, C., et al.: Hard carbon microspheres: potassium-ion anode versus sodium-ion anode. Adv. Energy Mater. 6, 1501874 (2016)
Song, H., Li, N., Cui, H., et al.: Enhanced capability and cyclability of SnO2–graphene oxide hybrid anode by firmly anchored SnO2 quantum dots. J. Mater. Chem. A 1, 7558–7562 (2013)
Fang, Y., Xiao, L., Ai, X., et al.: Hierarchical carbon framework wrapped Na3V2(PO4)3 as a superior high-rate and extended lifespan cathode for sodium-ion batteries. Adv. Mater. 27, 5895–5900 (2015)
Bruce, P.G., Scrosati, B., Tarascon, J.M.: Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 47, 2930–2946 (2008)
Szczech, J.R., Jin, S.: Nanostructured silicon for high capacity lithium battery anodes. Energy Environ. Sci. 4, 56–72 (2011)
Zhu, Y., Yin, Y., Yang, X., et al.: Transformation of rusty stainless-steel meshes into stable, low-cost, and binder-free cathodes for high-performance potassium-ion batteries. Angew. Chem. Int. Ed. 56, 7881–7885 (2017)
Wang, X., Xu, X., Niu, C., et al.: Earth abundant Fe/Mn-based layered oxide interconnected nanowires for advanced K-ion full batteries. Nano Lett. 17, 544–550 (2016)
Xiao, N., McCulloch, W.D., Wu, Y.: Reversible dendrite-free potassium plating and stripping electrochemistry for potassium secondary batteries. J. Am. Chem. Soc. 139, 9475–9478 (2017)
Jiang, X., Zhang, T., Yang, L., et al.: A Fe/Mn based Prussian blue analogue as a K-rich cathode material for potassium-ion batteries. ChemElectroChem 4, 2237–2242 (2017)
Acknowledgements
This work was financially supported by the Ministry of Science and Technology of the People’s Republic of China (Grant Nos. 2016YFB0100103 and 2017YFA0206704), the National Program on Key Basic Research Project of China (Grant No. 2014CB932300), the Technology and Industry for National Defence of the People’s Republic of China (Grant No. JCKY2016130B010), the National Natural Science Foundation of China (Grant Nos. 51522101, 51471075, 51631004 and 51401084) and the China Postdoctoral Science Foundation (Grant No. 2016M601395).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, YH., Yang, X., Sun, T. et al. Recent Progresses and Prospects of Cathode Materials for Non-aqueous Potassium-Ion Batteries. Electrochem. Energ. Rev. 1, 548–566 (2018). https://doi.org/10.1007/s41918-018-0019-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41918-018-0019-7