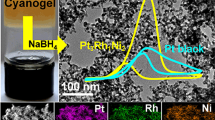
Abstract
Over the past several decades, extensive efforts have been undertaken to find methods to synthesize advanced electrocatalysts that possess rationally controllable sizes, shapes, crystallinities, compositions and structures for efficient energy conversion technologies. Of these methods, the one-pot seedless synthetic method in aqueous solution at ambient temperature has attracted extensive attention from researchers because it is a simple, inexpensive, energy-efficient, safe and less toxic method for the synthesis of electrocatalytic nanomaterials. In this review, recent developments in one-pot seedless synthetic strategies for the design of various structures of Au, Pt, Pd, Ag and multimetallic nanocrystals in aqueous solutions at ambient temperatures will be introduced, primarily focusing on the structure–electrocatalytic performance relationships of the as-prepared metal nanocrystals. Current challenges and outlooks for future research directions will also be provided in this promising research field.
Graphical Abstract


Source: a–f Reproduced with permission from Ref. [60]

Source: a–d Reproduced with permission from Ref. [61]

Source: a–g Reproduced with permission from Ref. [102]

Source: a Reproduced with permission from Ref. [106]

Source: a–b Reproduced with permission from Ref. [108]

Source: a–f Reproduced with permission from Ref. [112]
Similar content being viewed by others
References
Gasteiger, H.A., Marković, N.M.: Just a dream—or future reality? Science 324, 48–49 (2009)
Chu, S., Majumdar, A.: Opportunities and challenges for a sustainable energy future. Nature 488, 294–303 (2012)
Debe, M.K.: Electrocatalyst approaches and challenges for automotive fuel cells. Nature 486, 43–51 (2012)
Hwang, J., Rao, R.R., Giordano, L., et al.: Perovskites in catalysis and electrocatalysis. Science 358, 751–756 (2017)
Jiao, Y., Zheng, Y., Jaroniec, M., et al.: Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 44, 2060–2086 (2015)
Wang, Z.L., Li, C., Yamauchi, Y.: Nanostructured nonprecious metal catalysts for electrochemical reduction of carbon dioxide. Nano Today 11, 373–391 (2016)
Jin, H., Guo, C., Liu, X., et al.: Emerging two-dimensional nanomaterials for electrocatalysis. Chem. Rev. (2018). https://doi.org/10.1021/acs.chemrev.1027b00689
Adzic, R.R., Zhang, J., Sasaki, K., et al.: Platinum monolayer fuel cell electrocatalysts. Top. Catal. 4(6), 249–262 (2007)
Koenigsmann, C., Wong, S.S.: One-dimensional noble metal electrocatalysts: a promising structural paradigm for direct methanol fuel cells. Energy Environ. Sci. 4, 1161–1176 (2011)
Liang, Y., Li, Y., Wang, H., et al.: Strongly coupled inorganic/nanocarbon hybrid materials for advanced electrocatalysis. J. Am. Chem. Soc. 135, 2013–2036 (2013)
Zhao, Y., Liu, B., Pan, L., et al.: 3D nanostructured conductive polymer hydrogels for high-performance electrochemical devices. Energy Environ. Sci. 6, 2856–2870 (2013)
Faber, M.S., Jin, S.: Earth-abundant inorganic electrocatalysts and their nanostructures for energy conversion applications. Energy Environ. Sci. 7, 3519–3542 (2014)
Tang, J., Liu, J., Torad, N.L., et al.: Tailored design of functional nanoporous carbon materials toward fuel cell applications. Nano Today 9, 305–323 (2014)
Zhang, Z., Liu, J., Gu, J., et al.: An overview of metal oxide materials as electrocatalysts and supports for polymer electrolyte fuel cells. Energy Environ. Sci. 7, 2535–2558 (2014)
Chia, X., Eng, A.Y.S., Ambrosi, A., et al.: Electrochemistry of nanostructured layered transition-metal dichalcogenides. Chem. Rev. 115, 11941–11966 (2015)
Gawande, M.B., Goswami, A., Asefa, T., et al.: Core-shell nanoparticles: synthesis and applications in catalysis and electrocatalysis. Chem. Soc. Rev. 44, 7540–7590 (2015)
Liu, H.L., Nosheen, F., Wang, X.: Noble metal alloy complex nanostructures: controllable synthesis and their electrochemical property. Chem. Soc. Rev. 44, 3056–3078 (2015)
Lai, J., Nsabimana, A., Luque, R., et al.: 3D porous carbonaceous electrodes for electrocatalytic applications. Joule 2, 76–93 (2018)
Hunter, B.M., Gray, H.B., Müller, A.M.: Earth-abundant heterogeneous water oxidation catalysts. Chem. Rev. 116, 14120–14136 (2016)
Zhou, M., Wang, H.L., Guo, S.: Towards high-efficiency nanoelectrocatalysts for oxygen reduction through engineering advanced carbon nanomaterials. Chem. Soc. Rev. 45, 1273–1307 (2016)
Zhu, C., Li, H., Fu, S., et al.: Highly efficient nonprecious metal catalysts towards oxygen reduction reaction based on three-dimensional porous carbon nanostructures. Chem. Soc. Rev. 45, 517–531 (2016)
Zhu, D.D., Liu, J.L., Qiao, S.Z.: Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide. Adv. Mater. 28, 3423–3452 (2016)
Yan, D., Li, Y., Huo, J., et al.: Defect chemistry of nonprecious-metal electrocatalysts for oxygen reactions. Adv. Mater. 29, 1606459 (2017)
Zhu, C., Fu, S., Shi, Q., et al.: Single-atom electrocatalysts. Angew. Chem. Int. Ed. 56, 13944–13960 (2017)
Bianchini, C., Shen, P.K.: Palladium-based electrocatalysts for alcohol oxidation in half cells and in direct alcohol fuel cells. Chem. Rev. 109, 4183–4206 (2009)
Chen, A., Holt-Hindle, P.: Platinum-based nanostructured materials: synthesis, properties, and applications. Chem. Rev. 110, 3767–3804 (2010)
Wang, D., Li, Y.: Bimetallic nanocrystals: liquid-phase synthesis and catalytic applications. Adv. Mater. 23, 1044–1060 (2011)
Hu, S., Wang, X.: Ultrathin nanostructures: smaller size with new phenomena. Chem. Soc. Rev. 42, 5577–5594 (2013)
Wu, J., Yang, H.: Platinum-based oxygen reduction electrocatalysts. Acc. Chem. Res. 46, 1848–1857 (2013)
Kang, Y., Yang, P., Markovic, N.M., et al.: Shaping electrocatalysis through tailored nanomaterials. Nano Today 11, 587–600 (2016)
Lv, H., Li, D., Strmcnik, D., et al.: Recent advances in the design of tailored nanomaterials for efficient oxygen reduction reaction. Nano Energy 29, 149–165 (2016)
Seh, Z.W., Kibsgaard, J., Dickens, C.F. et .al.: Combining theory and experiment in electrocatalysis: Insights into materials design. Science 355, 146–157 (2017)
Lai, J., Guo, S.: Design of ultrathin Pt-based multimetallic nanostructures for efficient oxygen reduction electrocatalysis. Small 13, 1702156 (2017)
Chen, A., Ostrom, C.: Palladium-based nanomaterials: synthesis and electrochemical applications. Chem. Rev. 115, 11999–12044 (2015)
Wang, Y.J., Zhao, N., Fang, B., et al.: Carbon-supported Pt-based alloy electrocatalysts for the oxygen reduction reaction in polymer electrolyte membrane fuel cells: particle size, shape, and composition manipulation and their impact to activity. Chem. Rev. 115, 3433–3467 (2015)
Guo, S., Wang, E.: Noble metal nanomaterials: controllable synthesis and application in fuel cells and analytical sensors. Nano Today 6, 240–264 (2011)
Wu, B., Zheng, N.: Surface and interface control of noble metal nanocrystals for catalytic and electrocatalytic applications. Nano Today 8, 168–197 (2013)
Lai, J., Luque, R., Xu, G.: Recent advances in the synthesis and electrocatalytic applications of platinum-based bimetallic alloy nanostructures. ChemCatChem 7, 3206–3228 (2015)
You, H., Yang, S., Ding, B., et al.: Synthesis of colloidal metal and metal alloy nanoparticles for electrochemical energy applications. Chem. Soc. Rev. 42, 2880–2904 (2013)
Xu, Y., Zhang, B.: Recent advances in porous Pt-based nanostructures: synthesis and electrochemical applications. Chem. Soc. Rev. 43, 2439–2450 (2014)
Guo, S., Zhang, S., Sun, S.: Tuning nanoparticle catalysis for the oxygen reduction reaction. Angew.Chem. Int. Ed. 52, 8526–8544 (2013)
Zhang, L., Niu, W., Gao, W., et al.: Synthesis of convex hexoctahedralpalladium@gold core–shell nanocrystals with 431 high-index facets with remarkable electrochemiluminescence activities. ACS Nano 8, 5953–5958 (2014)
Lai, J., Niu, W., Li, S., et al.: Concave and duck web-like platinum nanopentagon with enhanced electrocatalytic properties for formic acid oxidation. J. Mater. Chem. A4, 807–812 (2016)
Wu, F., Lai, J., Zhang, l, et al.: Hierarchical concave layered triangular PtCu alloy nanostructures: rational integration of dendritic nanostructures for efficient formic acid electrooxidation. Nanoscale 10, 9369–9375 (2018)
Lai, J., Niu, W., Qi, W., et al.: A platinum highly concave cube with one leg on each vertex as an advanced nanocatalyst for electrocatalytic applications. ChemCatChem 7, 1064–1069 (2015)
Lai, J., Huang, B., Tang, Y., et al.: Barrier-free interface electron transfer on PtFe-Fe2C janus-like nanoparticles boosts oxygen catalysis. Chem 4, 1153–1166 (2018)
Zhu, C., Du, D., Eychmüller, A., et al.: Engineering ordered and nonordered porous noble metal nanostructures: synthesis, assembly, and their applications in electrochemistry. Chem. Rev. 115, 8896–8943 (2015)
Gilroy, K.D., Ruditskiy, A., Peng, H.C., et al.: Bimetallic nanocrystals: syntheses, properties, and applications. Chem. Rev. 116, 10414–10472 (2016)
Ling, T., Wang, J.J., Zhang, H., et al.: Freestanding ultrathin metallic nanosheets: materials, synthesis, and applications. Adv. Mater. 27, 5396–5402 (2015)
Malgras, V., Ataee-Esfahani, H., Wang, H., et al.: Nanoarchitectures for mesoporous metals. Adv. Mater. 28, 993–1010 (2016)
Wang, W., Lei, B., Guo, S.: Engineering multimetallic nanocrystals for highly efficient oxygen reduction catalysts. Adv. Energy Mater. 6, 1600236 (2016)
Wang, W., Lv, F., Lei, B., et al.: Tuning nanowires and nanotubes for efficient fuel-cell electrocatalysis. Adv. Mater. 28, 10117–10141 (2016)
Xia, Y., Xiong, Y., Lim, B., et al.: Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics? Angew. Chem. Int. Ed. 48, 60–103 (2009)
Lim, B., Jiang, M., Tao, J., et al.: Shape-controlled synthesis of Pd nanocrystals in aqueous solutions. Adv. Funct. Mater. 19, 189–200 (2009)
Lai, J., Niu, W., Luque, R., et al.: Solvothermal synthesis of metal nanocrystals and their applications. Nano Today 10, 240–267 (2015)
Hong, J.W., Kim, Y., Wi, D.H., et al.: Ultrathin free-standing ternary-alloy nanosheets. Angew. Chem. Int. Ed. 55, 2753–2758 (2016)
Duan, H., Wang, D., Li, Y.: Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 44, 5778–5792 (2015)
Niu, W., Li, Z.-Y., Shi, L., et al.: Seed-mediated growth of nearly monodisperse palladium nanocubes with controllable sizes. Cryst. Growth. Des. 8, 4440–4444 (2008)
Zeng, J., Zhang, Q., Chen, J., et al.: A comparison study of the catalytic properties of Au-based nanocages, nanoboxes, and nanoparticles. Nano Lett. 10, 30–35 (2009)
Pedireddy, S., Lee, H.K., Tjiu, W.W., et al.: One-step synthesis of zero-dimensional hollow nanoporous gold nanoparticles with enhanced methanol electrooxidation performance. Nat. Commun. 5, 4947 (2014)
Ma, Y., Kuang, Q., Jiang, Z., et al.: Synthesis of trisoctahedral gold nanocrystals with exposed high-index facets by a facile chemical method. Angew. Chem. Int. Ed. 47, 8901–8904 (2008)
Zhang, L., Niu, W., Xu, G.: Synthesis and applications of noble metal nanocrystals with high-energy facets. Nano Today 7, 586–605 (2012)
Quan, Z., Wang, Y., Fang, J.: High-index faceted noble metal nanocrystals. Acc. Chem. Res. 46, 191–202 (2012)
Chen, H., Shao, L., Li, Q., et al.: Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 42, 2679–2724 (2013)
Bao, D., Zhang, Q., Meng, F.L., et al.: Electrochemical reduction of N2 under ambient conditions for artificial N2 fixation and renewable energy storage using N2/NH3 cycle. Adv. Mater. 29, 1604799 (2017)
Shi, Y., Wang, J., Wang, C., et al.: Hot electron of Au nanorods activates the electrocatalysis of hydrogen evolution on MoS2 nanosheets. J. Am. Chem. Soc. 137, 7365–7370 (2015)
Katz-Boon, H., Walsh, M., Dwyer, C., et al.: Stability of crystal facets in gold nanorods. Nano Lett. 15, 1635–1641 (2015)
Jana, N.R.: Gram-scale synthesis of soluble, near-monodisperse gold nanorods and other anisotropic nanoparticles. Small 1, 875–882 (2005)
Lai, J., Zhang, L., Niu, W., et al.: One-pot synthesis of gold nanorods using binary surfactant systems with improved monodispersity, dimensional tunability and plasmon resonance scattering properties. Nanotechnology 25, 125601–125605 (2014)
Zhu, C., Peng, H.C., Zeng, J., et al.: Facile synthesis of gold wavy nanowires and investigation of their growth mechanism. J. Am. Chem. Soc. 134, 20234–20237 (2012)
Lai, J., Zhang, L., Qi, W., et al.: Facile synthesis of porous PtM (M = Cu, Ni) nanowires and their application as efficient electrocatalysts for methanol electrooxidation. ChemCatChem 6, 2253–2257 (2014)
Liang, H.W., Cao, X., Zhou, F., et al.: A free-standing Pt-nanowire membrane as a highly stable electrocatalyst for the oxygen reduction reaction. Adv. Mater. 23, 1467–1471 (2011)
Wu, H., Li, H., Zhai, Y., et al.: Facile synthesis of free-standing Pd-based nanomembranes with enhanced catalytic performance for methanol/ethanol oxidation. Adv. Mater. 24, 1594–1597 (2012)
Wu, H., He, H., Zhai, Y., et al.: A facile and general preparation of high-performance noble-metal-based free-standing nanomembranes by a reagentless interfacial self-assembly strategy. Nanoscale 4, 6974–6980 (2012)
Chen, L., Ji, F., Xu, Y., et al.: High-yield seedless synthesis of triangular gold nanoplates through oxidative etching. Nano Lett. 14, 7201–7206 (2014)
Zhang, J., Du, J., Han, B., et al.: Sonochemical formation of single-crystalline gold nanobelts. Angew. Chem. Int. Ed. 45, 1116–1119 (2006)
Chen, J., Lim, B., Lee, E.P., et al.: Shape-controlled synthesis of platinum nanocrystals for catalytic and electrocatalytic applications. Nano Today 4, 81–95 (2009)
Oezaslan, M., Hasché, F., Strasser, P.: Pt-Based core–shell catalyst architectures for oxygen fuel cell electrodes. J. Phys. Chem. Lett. 4, 3273–3291 (2013)
Jung, N., Chung, D.Y., Ryu, J., et al.: Pt-based nanoarchitecture and catalyst design for fuel cell applications. Nano Today 9, 433–456 (2014)
Peng, Z., Yang, H.: Designer platinum nanoparticles: control of shape, composition in alloy, nanostructure and electrocatalytic property. Nano Today 4, 143–164 (2009)
Stephens, I.E.L., Bondarenko, A.S., Gronbjerg, U., et al.: Understanding the electrocatalysis of oxygen reduction on platinum and its alloys. Energy Environ. Sci. 5, 6744–6762 (2012)
Wang, Y.J., Long, W., Wang, L., et al.: Unlocking the door to highly active ORR catalysts for PEMFC applications: polyhedron-engineered Pt-based nanocrystals. Energy Environ. Sci. 11, 258–275 (2017)
Lim, B., Xia, Y.: Metal nanocrystals with highly branched morphologies. Angew. Chem. Int. Ed. 50, 76–85 (2011)
Guo, S., Dong, S., Wang, E.: Ultralong Pt-on-Pd bimetallic nanowires with nanoporous surface: nanodendritic structure for enhanced electrocatalytic activity. Chem. Commun. 46, 1869–1871 (2010)
Lim, B., Jiang, M., Camargo, P.H.C., et al.: Pd-Pt bimetallic nanodendrites with high activity for oxygen reduction. Science 324, 1302–1305 (2009)
Wang, L., Yamauchi, Y.: Block copolymer mediated synthesis of dendritic platinum nanoparticles. J. Am. Chem. Soc. 131, 9152–9153 (2009)
Song, Y., Dorin, R.M., Garcia, R.M., et al.: Synthesis of platinum nanowheels using a bicellar template. J. Am. Chem. Soc. 130, 12602–12603 (2008)
Bu, L., Zhang, N., Guo, S., et al.: Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 354, 1410–1414 (2016)
Saleem, F., Zhang, Z., Xu, B., et al.: Ultrathin Pt–Cu nanosheets and nanocones. J. Am. Chem. Soc. 135, 18304–18307 (2013)
Antolini, E.: Palladium in fuel cell catalysis. Energy Environ. Sci. 2, 915–931 (2009)
Liao, F., Lo, T.W.B., Tsang, S.C.E.: Recent developments in palladium-based bimetallic catalysts. ChemCatChem 7, 1998–2014 (2015)
Zhang, L., Roling, L.T., Wang, X., et al.: Platinum-based nanocages with subnanometer-thick walls and well-defined, controllable facets. Science 349, 412–416 (2015)
Jin, M., Zhang, H., Xie, Z., et al.: Palladium nanocrystals enclosed by 100 and 111 facets in controlled proportions and their catalytic activities for formic acid oxidation. Energy Environ. Sci. 5, 6352–6357 (2012)
Sun, Y., Xia, Y.: Shape-controlled synthesis of gold and silver nanoparticles. Science 298, 2176–2179 (2002)
Chang, G., Oyama, M., Hirao, K.: Facile synthesis of monodisperse palladium nanocubes and the characteristics of self-assembly. Acta Mater. 55, 3453–3456 (2007)
Huang, X., Li, Y., Chen, Y., et al.: Palladium-based nanostructures with highly porous features and perpendicular pore channels as enhanced organic catalysts. Angew. Chem. Int. Ed. 52, 2520–2524 (2013)
Métraux, G.S., Mirkin, C.A.: Rapid thermal synthesis of silver nanoprisms with chemically tailorable thickness. Adv. Mater. 17, 412–415 (2005)
Yu, H., Zhang, Q., Liu, H., et al.: Thermal synthesis of silver nanoplates revisited: a modified photochemical process. ACS Nano 8, 10252–10261 (2014)
Zhang, Q., Li, N., Goebl, J., et al.: A systematic study of the synthesis of silver nanoplates: is citrate a “magic” reagent? J. Am. Chem. Soc. 133, 18931–18939 (2011)
Lee, Y.W., Kim, M., Kang, S.W., et al.: Polyhedral bimetallic alloy nanocrystals exclusively bound by 110 facets: Au–Pd rhombic dodecahedra. Angew. Chem. Int. Ed. 50, 3466–3470 (2011)
Huang, X., Li, Y., Chen, Y., et al.: Plasmonic and catalytic AuPd nanowheels for the efficient conversion of light into chemical energy. Angew. Chem. Int. Ed. 52, 6063–6067 (2013)
Huang, W., Kang, X., Xu, C., et al.: 2D PdAg alloy nanodendrites for enhanced ethanol electroxidation. Adv. Mater. 30, 1706962 (2018)
Wang, L., Yamauchi, Y.: Autoprogrammed synthesis of triple-layered Au@Pd@Pt core–shell nanoparticles consisting of aAu@Pd bimetallic core and nanoporous Pt shell. J. Am. Chem. Soc. 132, 13636–13638 (2010)
Wang, L., Nemoto, Y., Yamauchi, Y.: Direct synthesis of spatially-controlled Pt-on-Pd bimetallic nanodendrites with superior electrocatalytic activity. J. Am. Chem. Soc. 133, 9674–9677 (2011)
Ataee-Esfahani, H., Imura, M., Yamauchi, Y.: All-metal mesoporous nanocolloids: solution-phase synthesis of core–shell Pd@Pt nanoparticles with a designed concave surface. Angew. Chem. Int. Ed. 52, 13611–13615 (2013)
Wang, L., Yamauchi, Y.: Metallic nanocages: synthesis of bimetallic Pt–Pd hollow nanoparticles with dendritic shells by selective chemical etching. J. Am. Chem. Soc. 135, 16762–16765 (2013)
Liu, W., Herrmann, A.K., Bigall, N.C., et al.: Noble metal aerogels—synthesis, characterization, and application as electrocatalysts. Acc. Chem. Res. 48, 154–162 (2015)
Liu, W., Rodriguez, P., Borchardt, L., et al.: Bimetallic aerogels: high-performance electrocatalysts for the oxygen reduction reaction. Angew. Chem. Int. Ed. 52, 9849–9852 (2013)
Fu, S., Song, J., Zhu, C., et al.: Ultrafine and highly disorderedNi2Fe1nanofoams enabled highly efficient oxygen evolution reaction in alkaline electrolyte. Nano Energy 44, 319–326 (2018)
Scofield, M.E., Koenigsmann, C., Wang, L., et al.: Tailoring the composition of ultrathin, ternary alloy PtRuFe nanowires for the methanol oxidation reaction and formic acid oxidation reaction. Energy Environ. Sci. 8, 350–363 (2015)
Guo, S., Zhang, S., Sun, X., et al.: Synthesis of ultrathin FePtPd nanowires and their use as catalysts for methanol oxidation reaction. J. Am. Chem. Soc. 133, 15354–15357 (2011)
Li, S., Lai, J., Luque, R., et al.: Designed multimetallic Pd nanosponges with enhanced electrocatalytic activity for ethylene glycol and glycerol oxidation. Energy Environ. Sci. 9, 3097–3102 (2016)
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (51671003), the National Basic Research Program of China (No. 2016YFB0100201) and the Young Thousand Talented Program and China P Science Foundation (No. 2017M620494).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lai, J., Chao, Y., Zhou, P. et al. One-Pot Seedless Aqueous Design of Metal Nanostructures for Energy Electrocatalytic Applications. Electrochem. Energ. Rev. 1, 531–547 (2018). https://doi.org/10.1007/s41918-018-0018-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41918-018-0018-8