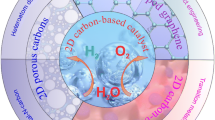
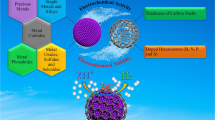
Abstract
Water splitting is an important approach for energy conversion to obtain hydrogen and oxygen. Apart from solar water splitting, electrochemical method plays a key role in the booming field, and it is urgent to develop novel and efficient catalysts to accelerate water splitting reaction. Recently, newly emerging self-supported materials, especially three dimensional (3D) carbon substrate electrochemical catalysts, have attracted great attention benefiting from their fantastic catalytic performances, such as large surface area, enhanced conductivity, tunable porosity, and so on. This review summarizes the outstanding materials used for hydrogen evolution reaction and oxygen evolution reaction. And catalysts that acted as both anode and cathode in two-electrode systems for overall water splitting are introduced systematically. The opportunities and challenges of 3D carbon substrate materials for electrochemical water splitting are proposed.
摘要
水分解制备氢气和氧气是能源转换的一种重要方法, 除光解水之外, 电解水也一直备受关注. 电解水过程中, 使用新型高效催化剂可以加快水分解并降低成本. 最近, 自支撑三维碳基材料由于其比表面积大、 导电性强、 耐酸碱性好等优点在电催化水分解领域引起研究者的广泛兴趣. 本文总结了近年来三维碳基材料在析氢、 析氧领域的研究现状, 重点介绍了其在双电极体系中分解水同时制氢气和氧气的研究进展, 指出了三维碳基材料在电解水领域存在的问题和未来的发展方向.
Similar content being viewed by others
References
Suh MP, Park HJ, Prasad TK, et al. Hydrogen storage in metal–organic frameworks. Chem Rev, 2012, 112: 782–835
Dai L, Xue Y, Qu L, et al. Metal-free catalysts for oxygen reduction reaction. Chem Rev, 2015, 115: 4823–4892
Dunn B, Kamath H, Tarascon JM. Electrical energy storage for the grid: a battery of choices. Science, 2011, 334: 928–935
Tachibana Y, Vayssieres L, Durrant JR. Artificial photosynthesis for solar water-splitting. Nat Photonics, 2012, 6: 511–518
Si H, Kang Z, Liao Q, et al. Design and tailoring of patterned ZnO nanostructures for energy conversion applications. Sci China Mater, 2017, 60: 793–810
Liu KH, Zhong HX, Li SJ, et al. Advanced catalysts for sustainable hydrogen generation and storage via hydrogen evolution and carbon dioxide/nitrogen reduction reactions. Prog Mater Sci, 2018, 92: 64–111
Chowdhury S, Balasubramanian R. Three-dimensional graphenebased macrostructures for sustainable energy applications and climate change mitigation. Prog Mater Sci, 2017, 90: 224–275
Mellmann D, Sponholz P, Junge H, et al. Formic acid as a hydrogen storage material–development of homogeneous catalysts for selective hydrogen release. Chem Soc Rev, 2016, 45: 3954–3988
Wang D, Marquard SL, Troian-Gautier L, et al. Interfacial deposition of Ru(II) bipyridine-dicarboxylate complexes by ligand substitution for applications in water oxidation catalysis. J Am Chem Soc, 2018, 140: 719–726
Sick T, Hufnagel AG, Kampmann J, et al. Oriented films of conjugated 2D covalent organic frameworks as photocathodes for water splitting. J Am Chem Soc, 2018, 140: 2085–2092
Zelny M, Kment S, Ctvrtlik R, et al. TiO2 nanotubes on transparent substrates: control of film microstructure and photoelectrochemical water splitting performance. Catalysts, 2018, 8: 25
Chen S, Takata T, Domen K. Particulate photocatalysts for overall water splitting. Nat Rev Mater, 2017, 2: 17050
Lee DK, Choi KS. Enhancing long-term photostability of BiVO4 photoanodes for solar water splitting by tuning electrolyte composition. Nat Energy, 2018, 3: 53–60
Naya SI, Kume T, Akashi R, et al. Red-light-driven water splitting by Au(core)–CdS(shell) half-cut nanoegg with heteroepitaxial junction. J Am Chem Soc, 2018, 140: 1251–1254
Du HY, Chen SC, Su XJ, et al. Redox-active ligand assisted multielectron catalysis: a case of CoIII complex as water oxidation catalyst. J Am Chem Soc, 2018, 140: 1557–1565
Chen YC, Hsu YK, Popescu R, et al. Au@Nb@HxK1-xNbO3 nanopeapods with near-infrared active plasmonic hot-electron injection for water splitting. Nat Commun, 2018, 9: 232
Han T, Chen Y, Tian G, et al. Hydrogenated TiO2/SrTiO3 porous microspheres with tunable band structure for solar-light photocatalytic H2 and O2 evolution. Sci China Mater, 2016, 59: 1003–1016
Huang Y, Yu Y, Xin Y, et al. Promoting charge carrier utilization by integrating layered double hydroxide nanosheet arrays with porous BiVO4 photoanode for efficient photoelectrochemical water splitting. Sci China Mater, 2017, 60: 193–207
Yu X, Hua T, Liu X, et al. Nickel-based thin film on multiwalled carbon nanotubes as an efficient bifunctional electrocatalyst for water splitting. ACS Appl Mater Interfaces, 2014, 6: 15395–15402
Zhong H, Wang J, Meng F, et al. In situ activating ubiquitous rust towards low-cost, efficient, free-standing, and recoverable oxygen evolution electrodes. Angew Chem Int Ed, 2016, 55: 9937–9941
Zhou J, Dou Y, Zhou A, et al. MOF template-directed fabrication of hierarchically structured electrocatalysts for efficient oxygen evolution reaction. Adv Energy Mater, 2017, 7: 1602643
Jia Y, Zhang L, Gao G, et al. A heterostructure coupling of exfoliated Ni-Fe hydroxide nanosheet and defective graphene as a bifunctional electrocatalyst for overall water splitting. Adv Mater, 2017, 29: 1700017
Zhu Y, Chen G, Xu X, et al. Enhancing electrocatalytic activity for hydrogen evolution by strongly coupled molybdenum nitride@ nitrogen-doped carbon porous nano-octahedrons. ACS Catal, 2017, 7: 3540–3547
Chen Y, Yu G, Chen W, et al. Highly active, nonprecious electrocatalyst comprising borophene subunits for the hydrogen evolution reaction. J Am Chem Soc, 2017, 139: 12370–12373
Dai Z, Geng H, Wang J, et al. Hexagonal-phase cobalt monophosphosulfide for highly efficient overall water splitting. ACS Nano, 2017, 11: 11031–11040
Blasco-Ahicart M, Soriano-López J, Carbó JJ, et al. Polyoxometalate electrocatalysts based on earth-abundant metals for efficient water oxidation in acidic media. Nat Chem, 2018, 10: 24–30
Li H, Tsai C, Koh AL, et al. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nat Mater, 2016, 15: 48–53
Xiao Z, Wang Y, Huang YC, et al. Filling the oxygen vacancies in Co3O4 with phosphorus: an ultra-efficient electrocatalyst for overall water splitting. Energy Environ Sci, 2017, 10: 2563–2569
Wang J, Xu F, Jin H, et al. Non-noble metal-based carbon composites in hydrogen evolution reaction: fundamentals to applications. Adv Mater, 2017, 29: 1605838
Roger I, Shipman MA, Symes MD. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat Rev Chem, 2017, 1: 0003
Voiry D, Yamaguchi H, Li J, et al. Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nat Mater, 2013, 12: 850–855
Zou X, Zhang Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem Soc Rev, 2015, 44: 5148–5180
Li C, Hou J, Wu Z, et al. Acid promoted Ni/NiO monolithic electrode for overall water splitting in alkaline medium. Sci China Mater, 2017, 60: 918–928
Liang HW, Brüller S, Dong R, et al. Molecular metal–Nx centres in porous carbon for electrocatalytic hydrogen evolution. Nat Commun, 2015, 6: 7992
Fan L, Liu PF, Yan X, et al. Atomically isolated nickel species anchored on graphitized carbon for efficient hydrogen evolution electrocatalysis. Nat Commun, 2016, 7: 10667
Sun C, Yang J, Dai Z, et al. Nanowires assembled from MnCo2O4 @C nanoparticles for water splitting and all-solid-state supercapacitor. Nano Res, 2016, 9: 1300–1309
Ma TY, Dai S, Qiao SZ. Self-supported electrocatalysts for advanced energy conversion processes. Mater Today, 2016, 19: 265–273
Shen K, Chen X, Chen J, et al. Development of MOF-derived carbon-based nanomaterials for efficient catalysis. ACS Catal, 2016, 6: 5887–5903
Lu Q, Hutchings GS, Yu W, et al. Highly porous non-precious bimetallic electrocatalysts for efficient hydrogen evolution. Nat Commun, 2015, 6: 6567
Kong X, Zhang C, Hwang SY, et al. Free-standing holey Ni(OH)2 nanosheets with enhanced activity for water oxidation. Small, 2017, 13: 1700334
Zhang B, Zheng X, Voznyy O, et al. Homogeneously dispersed multimetal oxygen-evolving catalysts. Science, 2016, 352: 333–337
Gou Y, Yang L, Liu Z, et al. Cu3Mo2O9 nanosheet array as a highefficiency oxygen evolution electrode in alkaline solution. Inorg Chem, 2018, 57: 1220–1225
Zhou W, Zhou K, Hou D, et al. Three-dimensional hierarchical frameworks based on MoS2 nanosheets self-assembled on graphene oxide for efficient electrocatalytic hydrogen evolution. ACS Appl Mater Interfaces, 2014, 6: 21534–21540
Lu X, Zhao C. Electrodeposition of hierarchically structured threedimensional nickel–iron electrodes for efficient oxygen evolution at high current densities. Nat Commun, 2016, 6: 6616
Wang S, Wang J, Zhu M, et al. Molybdenum-carbide-modified nitrogen-doped carbon vesicle encapsulating nickel nanoparticles: a highly efficient, low-cost catalyst for hydrogen evolution reaction. J Am Chem Soc, 2015, 137: 15753–15759
Kim Y, Lee S, Lee K, et al. Self-assembled plasmonic nanoparticles on vertically aligned carbon nanotube electrodes via thermal evaporation. ACS Appl Mater Interfaces, 2014, 6: 20423–20429
Ji D, Peng S, Lu J, et al. Design and synthesis of porous channelrich carbon nanofibers for self-standing oxygen reduction reaction and hydrogen evolution reaction bifunctional catalysts in alkaline medium. J Mater Chem A, 2017, 5: 7507–7515
Yang Y, Zhou K, Ma L, et al. Free-standing ternary NiWP film for efficient water oxidation reaction. Appl Surf Sci, 2018, 434: 871–878
Zhang Q, Yan D, Nie Z, et al. Iron-doped NiCoP porous nanosheet arrays as a highly efficient electrocatalyst for oxygen evolution reaction. ACS Appl Energy Mater, 2018, 1: 571–579
Zhang P, Li L, Nordlund D, et al. Dendritic core-shell nickel-ironcopper metal/metal oxide electrode for efficient electrocatalytic water oxidation. Nat Commun, 2018, 9: 381
Xu X, Du P, Chen Z, et al. An electrodeposited cobalt–selenidebased film as an efficient bifunctional electrocatalyst for full water splitting. J Mater Chem A, 2016, 4: 10933–10939
Sivanantham A, Ganesan P, Shanmugam S. Hierarchical NiCo2S4 nanowire arrays supported on Ni foam: an efficient and durable bifunctional electrocatalyst for oxygen and hydrogen evolution reactions. Adv Funct Mater, 2016, 26: 4661–4672
You B, Sun Y. Hierarchically porous nickel sulfide multifunctional superstructures. Adv Energy Mater, 2016, 6: 1502333
Wu Y, Liu Y, Li GD, et al. Efficient electrocatalysis of overall water splitting by ultrasmall NixCo3-xS4 coupled Ni3S2 nanosheet arrays. Nano Energy, 2017, 35: 161–170
Shi J, Hu J, Luo Y, et al. Ni3Se2 film as a non-precious metal bifunctional electrocatalyst for efficient water splitting. Catal Sci Technol, 2015, 5: 4954–4958
Lu W, Liu T, Xie L, et al. In situ derived CoB nanoarray: A highefficiency and durable 3D bifunctional electrocatalyst for overall alkaline water splitting. Small, 2017, 13: 1700805
Tian J, Cheng N, Liu Q, et al. Self-supported NiMo hollow nanorod array: an efficient 3D bifunctional catalytic electrode for overall water splitting. J Mater Chem A, 2015, 3: 20056–20059
He G, Zhang W, Deng Y, et al. Engineering pyrite-type bimetallic Ni-doped CoS2 nanoneedle arrays over a wide compositional range for enhanced oxygen and hydrogen electrocatalysis with flexible property. Catalysts, 2017, 7: 366
Han X, Tong X, Liu X, et al. Hydrogen evolution reaction on hybrid catalysts of vertical MoS2 nanosheets and hydrogenated graphene. ACS Catal, 2018, 8: 1828–1836
Guo X, Cao G, Ding F, et al. A bulky and flexible electrocatalyst for efficient hydrogen evolution based on the growth of MoS2 nanoparticles on carbon nanofiber foam. J Mater Chem A, 2015, 3: 5041–5046
Liu Y, Zhou X, Ding T, et al. 3D architecture constructed via the confined growth of MoS2 nanosheets in nanoporous carbon derived from metal–organic frameworks for efficient hydrogen production. Nanoscale, 2015, 7: 18004–18009
Wang J, Wang W, Ji L, et al. Highly dispersed Mo2C nanoparticles embedded in ordered mesoporous carbon for efficient hydrogen evolution. ACS Appl Energy Mater, 2018, 1: 736–743
Xiao J, Zhang Y, Zhang Z, et al. Self-supported biocarbon-fiber electrode decorated with molybdenum carbide nanoparticles for highly active hydrogen-evolution reaction. ACS Appl Mater Interfaces, 2017, 9: 22604–22611
Jiang P, Liu Q, Sun X. NiP2 nanosheet arrays supported on carbon cloth: an efficient 3D hydrogen evolution cathode in both acidic and alkaline solutions. Nanoscale, 2014, 6: 13440–13445
Wang L, Li Y, Xia M, et al. Ni nanoparticles supported on graphene layers: An excellent 3D electrode for hydrogen evolution reaction in alkaline solution. J Power Sources, 2017, 347: 220–228
Wang ZL, Hao XF, Jiang Z, et al. C and N hybrid coordination derived Co–C–N complex as a highly efficient electrocatalyst for hydrogen evolution reaction. J Am Chem Soc, 2015, 137: 15070–15073
Xiao X, Tao L, Li M, et al. Electronic modulation of transition metal phosphide via doping as efficient and pH-universal electrocatalysts for hydrogen evolution reaction. Chem Sci, 2018, 9: 1970–1975
Liu Q, Asiri AM, Sun X. Hematite nanorods array on carbon cloth as an efficient 3D oxygen evolution anode. Electrochem Commun, 2014, 49: 21–24
Wang J, Ma X, Qu F, et al. Fe-doped Ni2P nanosheet array for high-efficiency electrochemical water oxidation. Inorg Chem, 2017, 56: 1041–1044
Wang W, Liu D, Hao S, et al. High-efficiency and durable water oxidation under mild pH conditions: an iron phosphate–borate nanosheet array as a non-noble-metal catalyst electrode. Inorg Chem, 2017, 56: 3131–3135
Ye YJ, Zhang N, Liu XX. Amorphous NiFe(oxy)hydroxide nanosheet integrated partially exfoliated graphite foil for high efficiency oxygen evolution reaction. J Mater Chem A, 2017, 5: 24208–24216
Cao GL, Yan YM, Liu T, et al. Three-dimensional porous carbon nanofiber networks decorated with cobalt-based nanoparticles: A robust electrocatalyst for efficient water oxidation. Carbon, 2015, 94: 680–686
Dong Q, Wang Q, Dai Z, et al. MOF-derived Zn-doped CoSe2 as an efficient and stable free-standing catalyst for oxygen evolution reaction. ACS Appl Mater Interfaces, 2016, 8: 26902–26907
Zhang M, Dai Q, Zheng H, et al. Novel MOF-derived Co@N-C bifunctional catalysts for highly efficient Zn-air batteries and water splitting. Adv Mater, 2018, 30: 1705431
Hou Y, Cui S, Wen Z, et al. Strongly coupled 3D hybrids of Ndoped porous carbon nanosheet/CoNi alloy-encapsulated carbon nanotubes for enhanced electrocatalysis. Small, 2015, 11: 5940–5948
Zhang Z, Cao T, Liu S, et al. Substrate-induced synthesis of nitrogen-doped holey graphene nanocapsules for advanced metalfree bifunctional electrocatalysts. Part Part Syst Charact, 2017, 34: 1600207
Dong Q, Zhang Y, Dai Z, et al. Graphene as an intermediary for enhancing the electron transfer rate: A free-standing Ni3S2@graphene@Co9S8 electrocatalytic electrode for oxygen evolution reaction. Nano Res, 2018, 11: 1389–1398
Dong Q, Sun C, Dai Z, et al. Free-standing NiO@C nanobelt as an efficient catalyst for water splitting. ChemCatChem, 2016, 8: 3484–3489
Hou Y, Lohe MR, Zhang J, et al. Vertically oriented cobalt selenide/NiFe layered-double-hydroxide nanosheets supported on exfoliated graphene foil: an efficient 3D electrode for overall water splitting. Energy Environ Sci, 2016, 9: 478–483
Hou Y, Qiu M, Zhang T, et al. Efficient electrochemical and photoelectrochemical water splitting by a 3D nanostructured carbon supported on flexible exfoliated graphene foil. Adv Mater, 2017, 29: 1604480
Zhang Z, Liu S, Xiao J, et al. Fiber-based multifunctional nickel phosphide electrodes for flexible energy conversion and storage. J Mater Chem A, 2016, 4: 9691–9699
Zhang Z, Liu S, Xiao F, et al. Facile synthesis of heterostructured nickel/nickel oxide wrapped carbon fiber: flexible bifunctional gasevolving electrode for highly efficient overall water splitting. ACS Sustain Chem Eng, 2017, 5: 529–536
Wang J, Zhong H, Wang Z, et al. Integrated three-dimensional carbon paper/carbon tubes/cobalt-sulfide sheets as an efficient electrode for overall water splitting. ACS Nano, 2016, 10: 2342–2348
Sun C, Dong Q, Yang J, et al. Metal–organic framework derived CoSe2 nanoparticles anchored on carbon fibers as bifunctional electrocatalysts for efficient overall water splitting. Nano Res, 2016, 9: 2234–2243
Yang F, Zhao P, Hua X, et al. A cobalt-based hybrid electrocatalyst derived from a carbon nanotube inserted metal–organic framework for efficient water-splitting. J Mater Chem A, 2016, 4: 16057–16063
Ji D, Peng S, Fan L, et al. Thin MoS2 nanosheets grafted MOFsderived porous Co–N–C flakes grown on electrospun carbon nanofibers as self-supported bifunctional catalysts for overall water splitting. J Mater Chem A, 2017, 5: 23898–23908
Li M, Liu T, Bo X, et al. A novel flower-like architecture of Fe-Co@NC-functionalized ultra-thin carbon nanosheets as a highly efficient 3D bifunctional electrocatalyst for full water splitting. J Mater Chem A, 2017, 5: 5413–5425
Zhang Y, Xia X, Cao X, et al. Ultrafine metal nanoparticles/Ndoped porous carbon hybrids coated on carbon fibers as flexible and binder-free water splitting catalysts. Adv Energy Mater, 2017, 7: 1700220
Xue Y, Zuo Z, Li Y, et al. Graphdiyne-supported NiCo2S4 nanowires: A highly active and stable 3D bifunctional electrode material. Small, 2017, 13: 1700936
Malara F, Carallo S, Rotunno E, et al. A flexible electrode based on Al-doped nickel hydroxide wrapped around a carbon nanotube forest for efficient oxygen evolution. ACS Catal, 2017, 7: 4786–4795
Lai J, Li S, Wu F, et al. Unprecedented metal-free 3D porous carbonaceous electrodes for full water splitting. Energy Environ Sci, 2016, 9: 1210–1214
Zhang Z, Yi Z, Wang J, et al. Nitrogen-enriched polydopamine analogue-derived defect-rich porous carbon as a bifunctional metal-free electrocatalyst for highly efficient overall water splitting. J Mater Chem A, 2017, 5: 17064–17072
Acknowledgements
The work was supported by the National Natural Science Foundation of China (61525402, 61775095 and 5161101159), and Jiangsu Provincial Key Research and Development Plan (BE2017741).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xinglin Zhang received his BSc degree from the University of Jinan in 2011 and MSc degree from Nanjing University of Posts and Telecommunications in 2014. He is now a PhD candidate at the Institute of Advanced Materials (IAM), Nanjing Tech University under the supervision of Prof. Wei Huang and Prof. Xiaochen Dong. His research focuses on design and synthesis of nanomaterials for electrochemical water splitting.
Xiaochen Dong earned his PhD degree from Zhejiang University in 2007. Then he did the postdoctoral research at Nanyang Technological University (NTU), Singapore. In 2012, he joined the Institute of Advanced Materials (IAM), Nanjing Tech University as a full professor. He was supported by the National Science Fund for Distinguished Young Scholars in 2015. His research interests are nanomaterials for bio-optoelectronics, energy conversion and storage.
Wei Huang received his PhD degree from Peking University in 1992. In 1993, he started his postdoctoral research at National University of Singapore. In 2001, he moved to Fudan University where he founded the Institute of Advanced Material (IAM). In 2006, he was appointed vice president of Nanjing University of Posts and Telecommunications. He was elected as the Academician of Chinese Academy of Sciences in 2011. In 2012, he was appointed as the president of Nanjing Tech University. Now he is the Deputy President and Provost of the Northwestern Polytechnical University (NPU). His current research interests focus on flexible electronics.
Rights and permissions
About this article
Cite this article
Zhang, X., Shao, J., Huang, W. et al. Three dimensional carbon substrate materials for electrolysis of water. Sci. China Mater. 61, 1143–1153 (2018). https://doi.org/10.1007/s40843-018-9295-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-018-9295-8