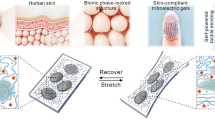
Abstract
Bio-integrated materials and devices can blur the interfaces between living and artificial systems. Microfluidics, bioelectronics, and engineered nanostructures, with close interactions with biology at the cellular or tissue levels, have already yielded a spectrum of new applications. Many new designs emerge, including of organ-on-a-chip systems, biodegradable implants, electroceutical devices, minimally invasive neuro-prosthetic tools, and soft robotics. In this review, we highlight a few recent advances of the fabrication and application of smart bio-hybrid systems, with a particular emphasis on the three-dimensional (3D) bio-integrated devices that mimic the 3D feature of tissue scaffolds. Moreover,neurons integrated with engineered nanostructures for wireless neuromodulation and dynamic neural output are briefly discussed. We also discuss the progress in the construction of cell-enabled soft robotics, where a tight coupling of the synthetic and biological parts is crucial for efficient function. Finally, we summarize the approaches for enhancing bio-integration with biomimetic micro- and nanostructures.

Similar content being viewed by others
References
Hyam, J. A.; Kringelbach, M. L.; Silburn, P. A.; Aziz, T. Z.; Green, A. L. The autonomic effects of deep brain stimulation—a therapeutic opportunity. Nat. Rev. Neurol. 2012, 8, 391–400.
Jackson, A.; Zimmermann, J. B. Neural interfaces for the brain and spinal cord—restoring motor function. Nat. Rev. Neurol. 2012, 8, 690–699.
Birmingham, K.; Gradinaru, V.; Anikeeva, P.; Grill, W. M.; Pikov, V.; McLaughlin, B.; Pasricha, P.; Weber, D.; Ludwig, K.; Famm, K. Bioelectronic medicines: A research roadmap. Nat. Rev. Drug Discov. 2014, 13, 399–400.
Fox, D. The shock tactics set to shake up immunology. Nature 2017, 545, 20–22.
Kozai, T. D. Y.; Jaquins-Gerstl, A. S.; Vazquez, A. L.; Michael, A. C.; Cui, X. T. Brain tissue responses to neural implants impact signal sensitivity and intervention strategies. ACS Chem. Neurosci. 2015, 6, 48–67.
Gunasekera, B.; Saxena, T.; Bellamkonda, R.; Karumbaiah, L. Intracortical recording interfaces: Current challenges to chronic recording function. ACS Chem. Neurosci. 2015, 6, 68–83.
Lacour, S. P.; Courtine, G.; Guck, J. Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater. 2016, 1, 16063.
Jeong, J. W.; Shin, G.; Park, S. I.; Yu, K. J.; Xu, L. Z.; Rogers, J. A. Soft materials in neuroengineering for hard problems in neuroscience. Neuron 2015, 86, 175–186.
Choi, S.; Lee, H.; Ghaffari, R.; Hyeon, T.; Kim, D. H. Recent advances in flexible and stretchable bio-electronic devices integrated with nanomaterials. Adv. Mater. 2016, 28, 4203–4218.
Green, R.; Abidian, M. R. Conducting polymers for neural prosthetic and neural interface applications. Adv. Mater. 2015, 27, 7620–7637.
Tian, B. Z.; Lieber, C. M. Synthetic nanoelectronic probes for biological cells and tissues. Annu. Rev. Anal. Chem. 2013, 6, 31–51.
Duan, X. J.; Fu, T. M.; Liu, J.; Lieber, C. M. Nanoelectronics-biology frontier: From nanoscopic probes for action potential recording in live cells to three-dimensional cyborg tissues. Nano Today 2013, 8, 351–373.
Zimmerman, J.; Parameswaran, R.; Tian, B. Z. Nanoscale semiconductor devices as new biomaterials. Biomater. Sci. 2014, 2, 619–626.
Cohen-Karni, T.; Langer, R.; Kohane, D. S. The smartest materials: The future of nanoelectronics in medicine. ACS Nano 2012, 6, 6541–6545.
Esch, E. W.; Bahinski, A.; Huh, D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 2015, 14, 248–260.
Guan, A.; Hamilton, P.; Wang, Y.; Gorbet, M.; Li, Z. Y.; Phillips, K. S. Medical devices on chips. Nat. Biomed. Eng. 2017, 1, 0045.
Feinberg, A. W. Biological soft robotics. Annu. Rev. Biomed. Eng. 2015, 17, 243–265.
Patino, T.; Mestre, R.; Sánchez, S. Miniaturized soft bio-hybrid robotics: A step forward into healthcare applications. Lab Chip 2016, 16, 3626–3630.
Dvir, T.; Timko, B. P.; Kohane, D. S.; Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 2011, 6, 13–22.
Bajaj, P.; Schweller, R. M.; Khademhosseini, A.; West, J. L.; Bashir, R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 2014, 16, 247–276.
Tian, B. Z.; Liu, J.; Dvir, T.; Jin, L. H.; Tsui, J. H.; Qing, Q.; Suo, Z. G.; Langer, R.; Kohane, D. S.; Lieber, C. M. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat. Mater. 2012, 11, 986–994.
Dai, X. C.; Zhou, W.; Gao, T.; Liu, J.; Lieber, C. M. Three-dimensional mapping and regulation of action potential propagation in nanoelectronics-innervated tissues. Nat. Nanotechnol. 2016, 11, 776–782.
Liu, J.; Xie, C.; Dai, X.; Jin, L.; Zhou, W.; Lieber, C. M. Multifunctional three-dimensional macroporous nanoelectronic networks for smart materials. Proc. Natl. Acad. Sci. USA 2013, 110, 6694–6699.
Feiner, R.; Engel, L.; Fleischer, S.; Malki, M.; Gal, I.; Shapira, A.; Shacham-Diamand, Y.; Dvir, T. Engineered hybrid cardiac patches with multifunctional electronics for online monitoring and regulation of tissue function. Nat. Mater. 2016, 15, 679–685.
Zhang, Y. H.; Zhang, F.; Yan, Z.; Ma, Q.; Li, X. L.; Huang, Y. G.; Rogers, J. A. Printing, folding and assembly methods for forming 3D mesostructures in advanced materials. Nat. Rev. Mater. 2017, 2, 17019.
Murphy, S. V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785.
Do, A. V.; Khorsand, B.; Geary, S. M.; Salem, A. K. 3D printing of scaffolds for tissue regeneration applications. Adv. Healthc. Mater. 2015, 4, 1742–1762.
Kong, Y. L.; Gupta, M. K.; Johnson, B. N.; McAlpine, M. C. 3D printed bionic nanodevices. Nano Today 2016, 11, 330–350.
Shin, S. R.; Farzad, R.; Tamayol, A.; Manoharan, V.; Mostafalu, P.; Zhang, Y. S.; Akbari, M.; Jung, S. M.; Kim, D.; Comotto, M. et al. A bioactive carbon nanotube-based ink for printing 2D and 3D flexible electronics. Adv. Mater. 2016, 28, 3280–3289.
Lind, J. U.; Busbee, T. A.; Valentine, A. D.; Pasqualini, F. S.; Yuan, H. Y.; Yadid, M.; Park, S. J.; Kotikian, A.; Nesmith, A. P.; Campbell, P. H. et al. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat. Mater. 2017, 16, 303–308.
Hwang, S. W.; Tao, H.; Kim, D. H.; Cheng, H. Y.; Song, J. K.; Rill, E.; Brenckle, M. A.; Panilaitis, B.; Won, S. M.; Kim, Y. S. et al. A physically transient form of silicon electronics. Science 2012, 337, 1640–1644.
Kang, S. K.; Murphy, R. K. J.; Hwang, S. W.; Lee, S. M.; Harburg, D. V.; Krueger, N. A.; Shin, J.; Gamble, P.; Cheng, H. Y.; Yu, S. et al. Bioresorbable silicon electronic sensors for the brain. Nature 2016, 530, 71–76.
Yu, K. J.; Kuzum, D.; Hwang, S. W.; Kim, B. H.; Juul, H.; Kim, N. H.; Won, S. M.; Chiang, K.; Trumpis, M.; Richardson, A. G. et al. Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex. Nat. Mater. 2016, 15, 782–791.
Zhang, B. Y.; Montgomery, M.; Chamberlain, M. D.; Ogawa, S.; Korolj, A.; Pahnke, A.; Wells, L. A.; Massé, S.; Kim, J.; Reis, L. et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat. Mater. 2016, 15, 669–678.
Fleischer, S.; Shapira, A.; Feiner, R.; Dvir, T. Modular assembly of thick multifunctional cardiac patches. Proc. Natl. Acad. Sci. USA 2017, 114, 1898–1903.
Cogan, S. F. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309.
Liu, J.; Fu, T. M.; Cheng, Z. G.; Hong, G. S.; Zhou, T.; Jin, L. H.; Duvvuri, M.; Jiang, Z.; Kruskal, P.; Xie, C. et al. Syringe-injectable electronics. Nat. Nanotechnol. 2015, 10, 629–636.
Xie, C.; Liu, J.; Fu, T. M.; Dai, X. C.; Zhou, W.; Lieber, C. M. Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes. Nat. Mater. 2015, 14, 1286–1292.
Luan, L.; Wei, X. L.; Zhao, Z. T.; Siegel, J. J.; Potnis, O.; Tuppen, C. A.; Lin, S. Q.; Kazmi, S.; Fowler, R. A.; Holloway, S. et al. Ultraflexible nanoelectronic probes form reliable, glial scar-free neural integration. Sci. Adv. 2017, 3, e1601966.
Chen, R.; Canales, A.; Anikeeva, P. Neural recording and modulation technologies. Nat. Rev. Mater. 2017, 2, 16093.
Tee, B. C. K.; Chortos, A.; Berndt, A.; Nguyen, A. K.; Tom, A.; McGuire, A.; Lin, Z. C.; Tien, K.; Bae, W. G.; Wang, H. L. et al. A skin-inspired organic digital mechanoreceptor. Science 2015, 350, 313–316.
Kim, C. K.; Adhikari, A.; Deisseroth, K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nat. Rev. Neurosci. 2017, 18, 222–235.
Rivnay, J.; Wang, H. L.; Fenno, L.; Deisseroth, K.; Malliaras, G. G. Next-generation probes, particles, and proteins for neural interfacing. Sci. Adv. 2017, 3, e1601649.
Carvalho-de-Souza, J. L.; Treger, J. S.; Dang, B.; Kent, S. B. H.; Pepperberg, D. R.; Bezanilla, F. Photosensitivity of neurons enabled by cell-targeted gold nanoparticles. Neuron 2015, 86, 207–217.
Eom, K.; Kim, J.; Choi, J. M.; Kang, T.; Chang, J. W.; Byun, K. M.; Jun, S. B.; Kim, S. J. Enhanced infrared neural stimulation using localized surface plasmon resonance of gold nanorods. Small 2014, 10, 3853–3857.
Yoo, S.; Hong, S.; Choi, Y.; Park, J. H.; Nam, Y. Photothermal inhibition of neural activity with near-infrared-sensitive nanotransducers. ACS Nano 2014, 8, 8040–8049.
Lyu, Y.; Xie, C.; Chechetka, S. A.; Miyako, E.; Pu, K. Semiconducting polymer nanobioconjugates for targeted photothermal activation of neurons. J. Am. Chem. Soc. 2016, 138, 9049–9052.
Jiang, Y. W.; Carvalho-de-Souza, J. L.; Wong, R. C. S.; Luo, Z. Q.; Isheim, D.; Zuo, X. B.; Nicholls, A. W.; Jung, I. W.; Yue, J. P.; Liu, D. J. et al. Heterogeneous silicon mesostructures for lipid-supported bioelectric interfaces. Nat. Mater. 2016, 15, 1023–1030.
Huang, H.; Delikanli, S.; Zeng, H.; Ferkey, D. M.; Pralle, A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat. Nanotechnol. 2010, 5, 602–606.
Stanley, S. A.; Gagner, J. E.; Damanpour, S.; Yoshida, M.; Dordick, J. S.; Friedman, J. M. Radio-wave heating of iron oxide nanoparticles can regulate plasma glucose in mice. Science 2012, 336, 604–608.
Chen, R.; Romero, G.; Christiansen, M. G.; Mohr, A.; Anikeeva, P. Wireless magnetothermal deep brain stimulation. Science 2015, 347, 1477–1480.
Munshi, R.; Qadri, S. M.; Zhang, Q.; Castellanos Rubio, I.; Del Pino, P.; Pralle, A. Magnetothermal genetic deep brain stimulation of motor behaviors in awake, freely moving mice. eLife 2017, 6, e27069.
Rus, D.; Tolley, M. T. Design, fabrication and control of soft robots. Nature 2015, 521, 467–475.
Feinberg, A. W.; Feigel, A.; Shevkoplyas, S. S.; Sheehy, S.; Whitesides, G. M.; Parker, K. K. Muscular thin films for building actuators and powering devices. Science 2007, 317, 1366–1370.
Nawroth, J. C.; Lee, H.; Feinberg, A. W.; Ripplinger, C. M.; McCain, M. L.; Grosberg, A.; Dabiri, J. O.; Parker, K. K. A tissue-engineered jellyfish with biomimetic propulsion. Nat. Biotechnol. 2012, 30, 792–797.
Cvetkovic, C.; Raman, R.; Chan, V.; Williams, B. J.; Tolish, M.; Bajaj, P.; Sakar, M. S.; Asada, H. H.; Saif, M. T. A.; Bashir, R. Three-dimensionally printed biological machines powered by skeletal muscle. Proc. Natl. Acad. Sci. USA 2014, 111, 10125–10130.
Raman, R.; Cvetkovic, C.; Bashir, R. A modular approach to the design, fabrication, and characterization of muscle-powered biological machines. Nat. Protoc. 2017, 12, 519–533.
Cvetkovic, C.; Rich, M. H.; Raman, R.; Kong, H.; Bashir, R. A 3D-printed platform for modular neuromuscular motor units. Microsyst. Nanoeng. 2017, 3, 17015.
Shin, S. R.; Shin, C.; Memic, A.; Shadmehr, S.; Miscuglio, M.; Jung, H. Y.; Jung, S. M.; Bae, H.; Khademhosseini, A.; Tang, X. S. et al. Aligned carbon nanotube-based flexible gel substrates for engineering biohybrid tissue actuators. Adv. Funct. Mater. 2015, 25, 4486–4495.
Raman, R.; Cvetkovic, C.; Uzel, S. G. M.; Platt, R. J.; Sengupta, P.; Kamm, R. D.; Bashir, R. Optogenetic skeletal muscle- powered adaptive biological machines. Proc. Natl. Acad. Sci. USA 2016, 113, 3497–3502.
Park, S. J.; Gazzola, M.; Park, K. S.; Park, S.; Di Santo, V.; Blevins, E. L.; Lind, J. U.; Campbell, P. H.; Dauth, S.; Capulli, A. K. et al. Phototactic guidance of a tissue-engineered soft-robotic ray. Science 2016, 353, 158–162.
Phan, L.; Kautz, R.; Leung, E. M.; Naughton, K. L.; Van Dyke, Y.; Gorodetsky, A. A. Dynamic materials inspired by cephalopods. Chem. Mater. 2016, 28, 6804–6816.
Pikul, J. H.; Li, S.; Bai, H.; Hanlon, R. T.; Cohen, I.; Shepherd, R. F. Stretchable surfaces with programmable 3D texture morphing for synthetic camouflaging skins. Science 2017, 358, 210–214.
Yu, C. J.; Li, Y. H.; Zhang, X.; Huang, X.; Malyarchuk, V.; Wang, S. D.; Shi, Y.; Gao, L.; Su, Y. W.; Zhang, Y. H. et al. Adaptive optoelectronic camouflage systems with designs inspired by cephalopod skins. Proc. Natl. Acad. Sci. USA 2014, 111, 12998–13003.
Li, J.; Celiz, A. D.; Yang, J.; Yang, Q.; Wamala, I.; Whyte, W.; Seo, B. R.; Vasilyev, N. V.; Vlassak, J. J.; Suo, Z. et al. Tough adhesives for diverse wet surfaces. Science 2017, 357, 378–381.
Zhao, Q.; Lee, D. W.; Ahn, B. K.; Seo, S.; Kaufman, Y.; Israelachvili, J. N.; Waite, J. H. Underwater contact adhesion and microarchitecture in polyelectrolyte complexes actuated by solvent exchange. Nat. Mater. 2016, 15, 407–412.
Gebbie, M. A.; Wei, W.; Schrader, A. M.; Cristiani, T. R.; Dobbs, H. A.; Idso, M.; Chmelka, B. F.; Waite, J. H.; Israelachvili, J. N. Tuning underwater adhesion with cation-π interactions. Nat. Chem. 2017, 9, 473–479.
Iturri, J.; Xue, L. J.; Kappl, M.; García-Fernández, L.; Barnes, W. J. P.; Butt, H. J.; del Campo, A. Torrent frog-inspired adhesives: Attachment to flooded surfaces. Adv. Funct. Mater. 2015, 25, 1499–1505.
Drotlef, D. M.; Stepien, L.; Kappl, M.; Barnes, W. J. P.; Butt, H. J.; del Campo, A. Insights into the adhesive mechanisms of tree frogs using artificial mimics. Adv. Funct. Mater. 2013, 23, 1137–1146.
Xue, L. J.; Sanz, B.; Luo, A. Y.; Turner, K. T.; Wang, X.; Tan, D.; Zhang, R.; Du, H.; Steinhart, M.; Mijangos, C. et al. Hybrid surface patterns mimicking the design of the adhesive toe pad of tree frog. ACS Nano 2017, 11, 9711–9719.
Lee, H.; Lee, B. P.; Messersmith, P. B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 2007, 448, 338–341.
Mahdavi, A.; Ferreira, L.; Sundback, C.; Nichol, J. W.; Chan, E. P.; Carter, D. J. D.; Bettinger, C. J.; Patanavanich, S.; Chignozha, L.; Ben-Joseph, E. et al. A biodegradable and biocompatible gecko-inspired tissue adhesive. Proc. Natl. Acad. Sci. USA 2008, 105, 2307–2312.
Frost, S. J.; Mawad, D.; Higgins, M. J.; Ruprai, H.; Kuchel, R.; Tilley, R. D.; Myers, S.; Hook, J. M.; Lauto, A. Gecko-inspired chitosan adhesive for tissue repair. NPG Asia Mater. 2016, 8, e280.
Luo, Z. Q.; Jiang, Y. W.; Myers, B. D.; Isheim, D.; Wu, J. S.; Zimmerman, J. F.; Wang, Z. G.; Li, Q. Q.; Wang, Y. C.; Chen, X. Q. et al. Atomic gold-enabled three-dimensional lithography for silicon mesostructures. Science 2015, 348, 1451–1455.
Cho, W. K.; Ankrum, J. A.; Guo, D. G.; Chester, S. A.; Yang, S. Y.; Kashyap, A.; Campbell, G. A.; Wood, R. J.; Rijal, R. K.; Karnik, R. et al. Microstructured barbs on the North American porcupine quill enable easy tissue penetration and difficult removal. Proc. Natl. Acad. Sci. USA 2012, 109, 21289–21294.
Yang, S. Y.; O’Cearbhaill, E. D.; Sisk, G. C.; Park, K. M.; Cho, W. K.; Villiger, M.; Bouma, B. E.; Pomahac, B.; Karp, J. M. A bio-inspired swellable microneedle adhesive for mechanical interlocking with tissue. Nat. Commun. 2013, 4, 1702.
Yi, J.; Wang, Y. C.; Jiang, Y. W.; Jung, I. W.; Liu, W. J.; De Andrade, V.; Xu, R. Q.; Parameswaran, R.; Peters, I. R.; Divan, R. et al. 3D calcite heterostructures for dynamic and deformable mineralized matrices. Nat. Commun. 2017, 8, 509.
Chen, Y. C.; Yang, H. T. Octopus-inspired assembly of nanosucker arrays for dry/wet adhesion. ACS Nano 2017, 11, 5332–5338.
Lee, H.; Um, D. S.; Lee, Y.; Lim, S.; Kim, H. J.; Ko, H. Octopus-inspired smart adhesive pads for transfer printing of semiconducting nanomembranes. Adv. Mater. 2016, 28, 7457–7465.
Baik, S.; Kim, D. W.; Park, Y.; Lee, T. J.; Ho Bhang, S.; Pang, C. A wet-tolerant adhesive patch inspired by protuberances in suction cups of octopi. Nature 2017, 546, 396–400.
Pan, F.; Gao, S.; Chen, C.; Song, C.; Zeng, F. Recent progress in resistive random access memories: Materials, switching mechanisms, and performance. Mater. Sci. Eng. R-Rep. 2014, 83, 1–59.
Sheridan, P. M.; Cai, F. X.; Du, C.; Ma, W.; Zhang, Z. Y.; Lu, W. D. Sparse coding with memristor networks. Nat. Nanotechnol. 2017, 12, 784–789.
van de Burgt, Y.; Lubberman, E.; Fuller, E. J.; Keene, S. T.; Faria, G. C.; Agarwal, S.; Marinella, M. J.; Alec Talin, A.; Salleo, A. A non-volatile organic electrochemical device as a low-voltage artificial synapse for neuromorphic computing. Nat. Mater. 2017, 16, 414–418.
Acknowledgements
Z.Q.L. acknowledges support from the National Natural Science Foundation of China (No. 81771974). B.Z.T. acknowledges a primary support from the University of Chicago Materials Research Science and Engineering Center, which is funded by the National Science Foundation under award number DMR-1420709. B.Z.T. also acknowledges support from the National Institutes of Health (No. NIH 1DP2NS101488).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Luo, Z., Weiss, D.E., Liu, Q. et al. Biomimetic approaches toward smart bio-hybrid systems. Nano Res. 11, 3009–3030 (2018). https://doi.org/10.1007/s12274-018-2004-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-018-2004-1