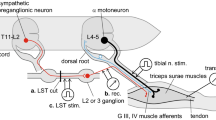
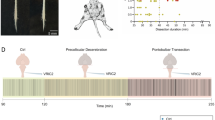
Abstract
Cholinergic networks have been shown to be involved in generation and modulation of the locomotor rhythmic pattern produced by the mammalian central pattern generators. Here, we show that changes in the endogenous levels of acetylcholine in the sacral segments of the isolated spinal cord of the neonatal rat modulate the locomotor-related output produced by stimulation of sacrocaudal afferents in muscarinic receptor-dependent mechanisms. Cholinergic components we found on sacral relay neurons with lumbar projections through the ventral and lateral funiculi are suggested to mediate this ascending cholinergic modulation. Our findings, possible mechanisms accounting for them, and their potential implications are discussed.




Similar content being viewed by others
References
Anglister L, Etlin A, Finkel E, Durrant AR, Lev-Tov A (2008) Cholinesterases in development and disease. Chem Biol Interact 175:92–100
Blivis D, Mentis GZ, O’Donovan MJ, Lev-Tov A (2007) Differential effects of opioids on sacrocaudal afferent pathways and central pattern generators in the neonatal rat spinal cord. J Neurophysiol 97:2875–2886
Carlin KP, Dai Y, Jordan LM (2006) Cholinergic and serotonergic excitation of ascending commissural neurons in the thoraco-lumbar spinal cord of the neonatal mouse. J Neurophysiol 95:1278–1284
Cherniak M, Etlin A, Federman N, Srauss I, Lev-Tov A (2011) Ventral horn clusters of sacral neurons are activated by α1 adrenoceptor agonists to produce “fast” rhythmic bursts in sacral and rostral lumbar motoneurons. J Mol Neurosci 45(suppl 1):S24
Cowley KC, Schmidt BJ (1994) A comparison of motor patterns induced by N-methyl-d-aspartate, acetylcholine and serotonin in the in vitro neonatal rat spinal cord. Neurosci Lett 171:147–150
Dietz V (2009) Body weight supported gait training: from laboratory to clinical setting. Brain Res Bull 78:I–VI
Dietz V, Duysens J (2000) Significance of load receptor input during locomotion: a review. Gait Posture 11:102–110
Dietz V, Harkema SJ (2004) Locomotor activity in spinal cord-injured persons. J Appl Physiol 96:1954–1960
Dietz V, Wirz M, Jensen L (1997) Locomotion in patients with spinal cord injuries. Phys Ther 77:508–516
Dietz V, Muller R, Colombo G (2002) Locomotor activity in spinal man: significance of afferent input from joint and load receptors. Brain 125:2626–2634
Duysens J, Pearson KG (1980) Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Res 187:321–332
Edgerton VR, Kim SJ, Ichiyama RM, Gerasimenko YP, Roy RR (2006) Rehabilitative therapies after spinal cord injury. J Neurotrauma 23:560–570
Edgerton VR, Courtine G, Gerasimenko YP, Lavrov I, Ichiyama RM, Fong AJ, Cai LL, Otoshi CK, Tillakaratne NJ, Burdick JW, Roy RR (2008) Training locomotor networks. Brain Res Rev 57:241–254
Eiden LE (1998) The cholinergic gene locus. J Neurochem 70:2227–2240
Etlin A, Blivis D, Ben-Zwi M, Lev-Tov A (2010) Long and short multifunicular projections of sacral neurons are activated by sensory input to produce locomotor activity in the absence of supraspinal control. J Neurosci 30:10324–10336
Etlin A, Finkel E, Mor Y, O’Donovan MJ, Anglister L, Lev-Tov A (2013) Characterization of sacral interneurons that mediate activation of locomotor pattern generators by sacrocaudal afferent input. J Neurosci 33:734–747
Finkel E, Etlin A, Cherniak M, Mor Y, Lev-Tov A Anglister L (2014) The neuroanatomical basis for cholinergic modulation of locomotor networks by sacral relay neurons with ascending lumbar projections. J Comp Neurol
Gabbay H, Lev-Tov A (2004) Alpha-1 adrenoceptor agonists generate a “fast” NMDA receptor-independent motor rhythm in the neonatal rat spinal cord. J Neurophysiol 92:997–1010
Grillner S, Rossignol S (1978) On the initiation of the swing phase of locomotion in chronic spinal cats. Brain Res 146:269–277
Grillner S, Zangger P (1979) On the central generation of locomotion in the low spinal cat. Exp Brain Res 34:241–261
Hubli M, Dietz V (2013) The physiological basis of neurorehabilitation—locomotor training after spinal cord injury. J Neuroeng Rehabil 10:5. doi:10.1186/1743-0003-10-5
Kiehn O (2006) Locomotor circuits in the mammalian spinal cord. Ann Rev Neurosci 29:279–306
Klein DA, Tresch MC (2010) Specificity of intramuscular activation during rhythms produced by spinal patterning systems in the in vitro neonatal rat with hindlimb attached preparation. J Neurophysiol 104:2158–2168
Koelle GB, Friedenwald JA (1949) A histochemical method for localizing cholinesterase activity. Proc Soc Exp Biol Med 1949(70):617–622
Lev-Tov A, Delvolve I, Kremer E (2000) Sacrocaudal afferents induce rhythmic efferent bursting in isolated spinal cords of neonatal rats. J Neurophysiol 83:888–894
Lev-Tov A, Etlin A, Blivis D (2010) Sensory-induced activation of pattern generators in the absence of supraspinal control. Ann NY Acad Sci 1198:54–62
Mandadi S, Whelan PJ (2009) A new method to study sensory modulation of locomotor networks by activation of thermosensitive cutaneous afferents using a hindlimb attached spinal cord preparation. J Neurosci Methods 182:255–259
Miles GB, Hartley R, Todd AJ, Brownstone RM (2007) Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci U S A 104:2448–2453
Miles GB, Sillar KT (2011) Neuromodulation of vertebrate locomotor control networks. Physiology 26:393–411
Mor Y, Lev-Tov A (2007) Analysis of rhythmic patterns produced by spinal neural networks. J Neurophysiol 98:2807–2817
Myers CP, Lewcock JW, Hanson MG, Gosgnach S, Aimone JB, Gage FH, Lee KF, Landmesser LT, Pfaff SL (2005) Cholinergic input is required during embryonic development to mediate proper assembly of spinal locomotor circuits. Neuron 46:37–49
Pearson KG (2004) Generating the walking gait: role of sensory feedback. Prog Brain Res 143:123–129
Prochazka A, Gillard D, Bennett DJ (1997) Positive force feedback control of muscles. J Neurophysiol 77:3226–3236
Smith JC, Feldman JL, Schmidt BJ (1988) Neural mechanisms generating locomotion studied in mammalian brain stem-spinal cord in vitro. Faseb J 2:2283–2288
Strauss I, Lev-Tov A (2003) Neural pathways between sacrocaudal afferents and lumbar pattern generators in neonatal rats. J Neurophysiol 89:773–784
Taylor P (1996) Anticholinesterase agents, in Goodman and Gillman’s. In: Hardman, J.G, Limbird, L.E, Molinoff, P.B., Ruddon, R.W, Gilman, A.G (eds) The pharmacologial basis of therapeutics The McGraw-Hill Companies, Inc., pp.161–174
Wernig A, Nanassy A, Muller S (1998) Maintenance of locomotor abilities following Laufband (treadmill) therapy in para- and tetraplegic persons: follow-up studies. Spinal Cord 36:744–749
Zagoraiou L, Akay T, Martin JF, Brownstone RM, Jessell TM, Miles GB (2009) A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron 64:645–662
Acknowledgments
The research was supported by grants from Israel Science Foundation (ISF) 1591/08, 1930/08, 491/12 to ALT, and ISF 685/01 and MDA to LA.
Conflict of interests
There are no known or potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Eran Finkel (deceased)
Rights and permissions
About this article
Cite this article
Etlin, A., Finkel, E., Cherniak, M. et al. The Motor Output of Hindlimb Innervating Segments of the Spinal Cord is Modulated by Cholinergic Activation of Rostrally Projecting Sacral Relay Neurons. J Mol Neurosci 53, 517–524 (2014). https://doi.org/10.1007/s12031-014-0351-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-014-0351-2