Abstract
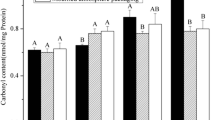
The objective of this study was to investigate the biochemical difference of pork under high oxygen modified atmosphere packaging and their contribution to meat tenderness and water holding capacity of pork during postmortem storage. Twelve longissimus dorsi muscles were randomly assigned to either high oxygen modified atmosphere packaging or vacuum packaging and stored for 1, 4, and 6 days at 4 °C. The carbonyl content, protein surface hydrophobicity, protein solubility, calpain activity, desmin degradation, tenderness, and water loss of pork were determined. Results showed that carbonyl content, protein surface hydrophobicity, and protein solubility were significantly affected (P < 0.05) by packaging method, while storage time did not significantly influence protein surface hydrophobicity and the solubility of sarcoplasmic protein (P > 0.05). Samples from high oxygen modified atmosphere packaging at 1 day showed greater intensity of intact 80 KDa calpain and lower intensity of autolyzed 76 KDa calpain product compared to samples from vacuum packaging (P < 0.05). Desmin degradation was significantly affected (P < 0.05) by packaging method and storage time, while their interaction presented no significance (P > 0.05). Higher intensity of intact desmin was observed in samples from high oxygen modified atmosphere packaging than vacuum packaging samples from 1 day of postmortem storage. Both packaging method and storage time showed significant effects (P < 0.05) on tenderness and water loss of pork muscle during postmortem storage. Changes in protein oxidation, calpain activation, and protein proteolysis of postmortem pork under high oxygen modified atmosphere packaging could help to explain decreased meat tenderness and increased centrifuge loss of pork.



Similar content being viewed by others
Abbreviations
- HiOx:
-
High oxygen modified atmosphere packaging
- VP:
-
Vacuum packaging
- WHC:
-
Water holding capacity
- WBSF:
-
Warner-Bratzler shear force
- LD:
-
Longissimus dorsi
- PM:
-
Packaging method
- ST:
-
Storage time
- PM × ST:
-
Interaction of packaging method and storage time
- LF-NMR:
-
Low-field nuclear magnetic resonance
- DNPH:
-
2,4-Dinitrophenylhydrazine
- BPB:
-
Bromophenol blue
References
Amici, A., Levine, R. L., Tsai, L., & Stadtman, E. R. (1989). Conversion of amino acid residues in proteins and amino acid homopolymers to carbonyl derivatives by metal-catalyzed oxidation reactions. Journal of Biological Chemistry, 264(6), 3341–3346.
Astruc, T., Marinova, P., Labas, R., Gatellier, P., & Santé-Lhoutellier, V. (2007). Detection and localization of oxidized proteins in muscle cells by fluorescence microscopy. Journal of Agricultural and Food Chemistry, 55(23), 9554–9558.
Bertram, H. C., Purslow, P. P., & Andersen, H. J. (2002). Relationship between meat structure, water mobility, and distribution: a low-field nuclear magnetic resonance study. Journal of Agricultural and Food Chemistry, 50(4), 824–829.
Burcham, P. C., & Kuhan, Y. T. (1996). Introduction of carbonyl groups into proteins by the lipid peroxidation product, malondialdehyde. Biochemical and Biophysical Research Communications, 220(3), 996–1001.
Carlin, K. R., Huff-Lonergan, E., Rowe, L. J., & Lonergan, S. M. (2006). Effect of oxidation, pH, and ionic strength on calpastatin inhibition of μ-and m-calpain. Journal of Animal Science, 84(4), 925–937.
Carr, H. Y., & Purcell, E. M. (1954). Effects of diffusion on free precession in nuclear magnetic resonance experiments. Physical Review, 94(3), 630–638.
Cayuela, J. M., Gil, M. D., Bañón, S., & Garrido, M. D. (2004). Effect of vacuum and modified atmosphere packaging on the quality of pork loin. European Food Research and Technology, 219(4), 316–320.
Chao, C. C., Ma, Y. S., & Stadtman, E. R. (1997). Modification of protein surface hydrophobicity and methionine oxidation by oxidative systems. Proceedings of the National Academy of Sciences, 94(7), 2969–2974.
Chelh, I., Gatellier, P., & Santé-Lhoutellier, V. (2006). Technical note: a simplified procedure for myofibril hydrophobicity determination. Meat Science, 74(4), 681–683.
Cheng, Q., & Sun, D. W. (2008). Factors affecting the water holding capacity of red meat products: a review of recent research advances. Critical Reviews in Food Science and Nutrition, 48(2), 137–159.
Choi, Y. M., Lee, S. H., Choe, J. H., Rhee, M. S., Lee, S. K., Joo, S. T., & Kim, B. C. (2010). Protein solubility is related to myosin isoforms, muscle fiber types, meat quality traits, and postmortem protein changes in porcine longissimus dorsi muscle. Livestock Science, 127(2), 183–191.
Delles, R. M., & Xiong, Y. L. (2014). The effect of protein oxidation on hydration and water-binding in pork packaged in an oxygen-enriched atmosphere. Meat Science, 97(2), 181–188.
Dikeman, M. E., Tuma, H J. & Beecher G. R. (1971). Bovine muscle tenderness as related to protein solubility. Journal of Food Science, 36(2), 190-193.
Fu, Q. Q., Liu, R., Zhang, W. G., Li, Y. P., Wang, J., & Zhou, G. H. (2015). Effects of different packaging systems on beef tenderness through protein modifications. Food and Bioprocess Technology, 8, 580–588.
Goll, D. E., Thompson, V. F., Li, H., Wei, W. E. I., & Cong, J. (2003). The calpain system. Physiological Reviews, 83(3), 731–801.
Huff-Lonergan, E., & Lonergan, S. M. (2005). Mechanisms of water-holding capacity of meat: the role of postmortem biochemical and structural changes. Meat Science, 71(1), 194–204.
Huff-Lonergan, E., Mitsuhashi, T., Beekman, D. D., Parrish, J. F. C., Olson, D. G., & Robson, R. M. (1996). Proteolysis of specific muscle structural proteins by mu-calpain at low pH and temperature is similar to degradation in postmortem bovine muscle. Journal of Animal Science, 74(5), 993–1008.
Huff-Lonergan, E., Zhang, W. G., & Lonergan, S. M. (2010). Biochemistry of postmortem muscle - lessons on mechanisms of meat tenderization. Meat Science, 86(1), 184–195.
Jongberg, S., Wen, J. Z., Tørngren, M. A., & Lund, M. N. (2014). Effect of high-oxygen atmosphere packaging on oxidative stability and sensory quality of two chicken muscles during chill storage. Food Packaging and Shelf Life, 1(1), 38–48.
Joo, S. T., Kauffman, R. G., Kim, B. C., & Park, G. B. (1999a). The relationship of sarcoplasmic and myofibrillar protein solubility to colour and water-holding capacity in porcine longissimus muscle. Meat Science, 52(3), 291–297.
Joo, S. T., Kauffman, R. G., Laack, R., Lee, S., & Kim, B. C. (1999b). Variations in rate of water loss as related to different types of post-rigor porcine musculature during storage. Journal of Food Science, 64(5), 865–868.
Kim, Y. H., Huff-Lonergan, E., Sebranek, J. G.. & Lonergan, S. M. (2010). High-oxygen modified atmosphere packaging system induces lipid and myoglobin oxidation and protein polymerization. Meat Science, 85(4), 759-767.
Koohmaraie, M., & Geesink, G. H. (2006). Contribution of postmortem muscle biochemistry to the delivery of consistent meat quality with particular focus on the calpain system. Meat Science, 74(1), 34–43.
Kristensen, L., & Purslow, P. P. (2001). The effect of ageing on the water-holding capacity of pork: role of cytoskeletal proteins. Meat Science, 58(1), 17–23.
Ladikos, D., & Lougovois, V. (1990). Lipid oxidation in muscle foods: a review. Food Chemistry, 35(4), 295–314.
Lametsch, R., Lonergan, S., & Huff-Lonergan, E. (2008). Disulfide bond within μ-calpain active site inhibits activity and autolysis. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 1784(9), 1215–1221.
Lindahl, G., Lagerstedt, Å., Ertbjerg, P., Sampels, S., & Lundström, K. (2010). Ageing of large cuts of beef loin in vacuum or high oxygen modified atmosphere—effect on shear force, calpain activity, desmin degradation and protein oxidation. Meat Science, 85(1), 160–166.
Liu, G., & Xiong, Y. L. (2000). Electrophoretic pattern, thermal denaturation, and in vitro digestibility of oxidized myosin. Journal of Agricultural and Food Chemistry, 48(3), 624–630.
Lund, M. N., Heinonen, M., Baron, C. P., & Estevez, M. (2011). Protein oxidation in muscle foods: a review. Molecular Nutrition & Food Research, 55(1), 83–95.
Lund, M. N., Lametsch, R., Hviid, M. S., Jensen, O. N., & Skibsted, L. H. (2007). High-oxygen packaging atmosphere influences protein oxidation and tenderness of porcine longissimus dorsi during chill storage. Meat Science, 77(3), 295–303.
McDonnell, C. K., Allen, P., Duggan, E., Arimi, J. M., Casey, E., Duane, G., & Lyng, J. G. (2013). The effect of salt and fibre direction on water dynamics, distribution and mobility in pork muscle: a low field NMR study. Meat Science, 95(1), 51–58.
Meiboom, S., & Gill, D. (1958). Modified spin-echo method for measuring nuclear relaxation times. Review of Scientific Instruments, 29(8), 688–691.
Melody, J. L., Lonergan, S. M., Rowe, L. J., Huiatt, T. W., Mayes, M. S., & Huff-Lonergan, E. (2004). Early postmortem biochemical factors influence tenderness and water-holding capacity of three porcine muscles. Journal of Animal Science, 82(4), 1195–1205.
Pearce, K. L., Rosenvold, K., Andersen, H. J., & Hopkins, D. L. (2011). Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes—a review. Meat Science, 89(2), 111–124.
Rowe, L. J., Maddock, K. R., Lonergan, S. M., & Huff-Lonergan, E. (2004). Oxidative environments decrease tenderization of beef steaks through inactivation of μ-calpain. Journal of Animal Science, 82(11), 3254–3266.
Santé-Lhoutellier, V., Aubry, L., & Gatellier, P. (2007). Effect of oxidation on in vitro digestibility of skeletal muscle myofibrillar proteins. Journal of Agricultural and Food Chemistry, 55(13), 5343–5348.
Santé-Lhoutellier, V., Engel, E., Aubry, L., & Gatellier, P. (2008). Effect of animal (lamb) diet and meat storage on myofibrillar protein oxidation and in vitro digestibility. Meat Science, 79(4), 777–783.
Sayre, R. N., & Briskey, E. J. (1963). Protein solubility as influenced by physiological conditions in the muscle. Journal of Food Science, 28(6), 675–679.
Schluter, A. R., Miller, M. F., Jones, D. K., Meade, M. K., Ramsey, C. B., & Patterson, L. L. (1994). Effects of distribution packaging method and storage time on the physical properties and retail display characteristics of pork. Meat Science, 37(2), 257–269.
Taylor, R. G., Geesink, G. H., Thompson, V. F., Koohmaraie, M., & Goll, D. E. (1995). Is Z-disk degradation responsible for postmortem tenderization? Journal of Animal Science, 73(5), 1351–1367.
Traore, S., Aubry, L., Gatellier, P., Przybylski, W., Jaworska, D., Kajak-Siemaszko, K., & Santé-Lhoutellier, V. (2012a). Higher drip loss is associated with protein oxidation. Meat Science, 90(1), 917–924.
Traore, S., Aubry, L., Gatellier, P., Przybylski, W., Jaworska, D., Kajak-Siemaszko, K., & Santé-Lhoutellier, V. (2012b). Effect of heat treatment on protein oxidation in pig meat. Meat Science, 91(1), 14–21.
Trout, G. R. (1988). Techniques for measuring water-binding capacity in muscle foods—a review of methodology. Meat Science, 23(4), 235–252.
Veiseth, E., Shackelford, S. D., Wheeler, T. L., & Koohmaraie, M. (2001). Effect of postmortem storage on mu-calpain and m-calpain in ovine skeletal muscle. Journal of Animal Science, 79(6), 1502–1508.
Wilson, G. G., & van Laack, R. L. J. M. (1999). Sarcoplasmic proteins influence water-holding capacity of pork myofibrils. Journal of the Science of Food and Agriculture, 79(13), 1939–1942.
Xiao, S., Zhang, W. G., Lee, E. J., Ma, C. W., & Ahn, D. U. (2011). Lipid and protein oxidation of chicken breast rolls as affected by dietary oxidation levels and packaging. Journal of Food Science, 76(4), C612–C617.
Xiong, Y. L. (2005). Role of myofibrillar proteins in water-binding in brine-enhanced meats. Food Research International, 38(3), 281–287.
Xue, M., Huang, F., Huang, M., & Zhou, G. H. (2012). Influence of oxidation on myofibrillar proteins degradation from bovine via μ-calpain. Food Chemistry, 134(1), 106–112.
Yin, Y., Zhang, W. G., Zhou, G. H., & Guo, B. (2014). Comparison of protein degradation, protein oxidation, and μ-calpain activation between pale, soft, and exudative and red, firm, and nonexudative pork during postmortem aging. Journal of Animal Science, 92(8), 3745–3752.
Zakrys-Waliwander, P. I., O’Sullivan, M. G., O’Neill, E. E., & Kerry, J. P. (2012). The effects of high oxygen modified atmosphere packaging on protein oxidation of bovine M. longissimus dorsi muscle during chilled storage. Food Chemistry, 131(2), 527–532.
Zhang, W. G., Lonergan, S. M., Gardner, M. A., & Huff-Lonergan, E. (2006). Contribution of postmortem changes of integrin, desmin and μ-calpain to variation in water holding capacity of pork. Meat Science, 74(3), 578–585.
Zhang, W. G., Marwan, A. H., Samaraweera, H., Lee, E. J., & Ahn, D. U. (2013a). Breast meat quality of broiler chickens can be affected by managing the level of nitric oxide. Poultry Science, 92, 3044–3049.
Zhang, W. G., Xiao, S., & Ahn, D. U. (2013b). Protein oxidation: basic principles and implications for meat quality. Critical Reviews in Food Science and Nutrition, 53(11), 1191–1201.
Acknowledgments
The authors would like to thank The National Natural Science Foundation of China (31271899) and The Ministry of Science and Technology of China (2012BAD28B03) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, L., Zhou, Gh. & Zhang, Wg. Effects of High Oxygen Packaging on Tenderness and Water Holding Capacity of Pork Through Protein Oxidation. Food Bioprocess Technol 8, 2287–2297 (2015). https://doi.org/10.1007/s11947-015-1566-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-015-1566-0