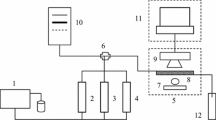
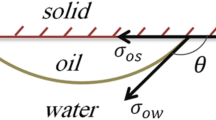
Abstract
The main production mechanism during water flooding of naturally fractured oil reservoirs is the spontaneous imbibition of water into matrix blocks and resultant displacement of oil into the fracture system. This is an efficient recovery process when the matrix is strongly water-wet. However, in mixed- to oil-wet reservoirs, secondary recovery from water flooding is often poor. Oil production can be improved by dissolving low concentrations of surfactants in the injected water. The surfactant alters the wettability of the reservoir rock, enhancing the spontaneous imbibition process. Our previous study revealed that the two main mechanisms responsible for the wettability alteration are ion-pair formation and adsorption of surfactant molecules through interactions with the adsorbed crude oil components on the rock surface. Based on the superior performance of surfactin, an anionic biosurfactant with two charged groups on the hydrophilic head, it was hypothesized that the wettability alteration process might be further improved through the use of dimeric or gemini surfactants, which have two hydrophilic head groups and two hydrophobic tails. We believe that when ion-pair formation is the dominant wettability alteration mechanism, wettability alteration in oil-wet cores can be improved by increasing the charge density on the head group(s) of the surfactant molecule since the ion-pair formation is driven by electrostatic interactions. At a concentration of 1.0 mmol L−1 a representative anionic gemini surfactant showed oil recoveries of up to 49% original oil-in-place (OOIP) from oil-wet sandstone cores, compared to 6 and 27% for sodium laureth sulfate and surfactin, respectively. These observations are consistent with our hypothesis.




Similar content being viewed by others
References
Allan J, Sun SQ (2003) Controls on recovery factor in fractured reservoirs: lessons learned from 100 fractured fields. Paper SPE 84590 presented at the SPE annual technical conference and exhibition. Denver, CO, USA
Roehl PO, Choquette PW (1985) Carbonate petroleum reservoirs. Springer-Verlag, New York
Austad T, Matre B, Milter J, Saevareid A, Oyno L (1998) Chemical flooding of oil reservoirs 8. Spontaneous oil expulsion from oil-and water-wet low permeable chalk material by imbibition of aqueous surfactant solutions. Colloids Surf A 137:117–129
Standnes DC (2001) Enhanced oil recovery from oil-wet carbonate rock by spontaneous imbibition of aqueous surfactant solutions, Ph.D. Thesis, Stavanger College, p 115
Standnes DC, Austad T (2000) Wettability Alteration in Chalk 2. Mechanism for wettability alteration from oil-wet to water-wet using surfactants. J Petroleum Sci Technol 28:123–143
Salehi M, Johnson SJ, Liang J-T (2008) Mechanistic study of wettability alteration using surfactants with applications in naturally fractured reservoirs. Langmuir 24:14099–14107
ASTM International (2007) ASTM D2896-07a standard test method for base number of petroleum products by potentiometric perchloric acid titration, in annual book of ASTM Standards. ASTM International, West Conshohocken
Fan T, Buckley JS (2007) Acid number measurements revisited. Soc Petroleum Eng J 12:496–500
ASTM International (2007) D664-07 standard test method for acid number of petroleum products by potentiometric titration. Annual Book of ASTM Standards. ASTM International, West Conshohocken
Hirasaki GJ, Rohan JA, Dubey ST, Niko H (1990) Wettability evaluation during restored state core analysis. Paper SPE 20506 presented at the SPE 65th annual technical conference and exhibition. New Orleans, pp 361–375
Li Y, Ye R-Q, Mu B-Z (2009) Influence of sodium ions on micelles of surfactin-C16 in solution. J Surf Deterg 12:31–36
Mukerjee P, Mysels KJ (1971) Critical micelle concentration of aqueous surfactant systems. US Department of Commerce, National Bureau of Standards, Washington, DC
Morrow NR, McCaffrey F (1978) Displacement studies in uniformly wetted porous media. In: Padday GF (ed) Wetting, spreading and adhesion. Academic Press, New York, pp 289–319
Acknowledgments
The authors would like to thank the US Department of Energy (DOE) for funding this work through Contract NO. DF-FC26-04NT15523. Thanks also to Mr. Gregory Bala and Ms. Sandra Fox at Idaho National Laboratory, Idaho Falls, for providing and characterizing surfactin, and to Dr. Wenyu Zhang (SINOPEC), visiting scholar at the Tertiary Oil Recovery Project, for acid number measurement. We extend our thanks to the Department of Chemical and Petroleum Engineering at the University of Kansas.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Salehi, M., Johnson, S.J. & Liang, JT. Enhanced Wettability Alteration by Surfactants with Multiple Hydrophilic Moieties. J Surfact Deterg 13, 243–246 (2010). https://doi.org/10.1007/s11743-010-1193-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-010-1193-8