Abstract
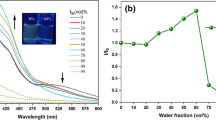
Exploration of novel organic luminophores that exhibit thermally activated delayed fluorescence (TADF) in the aggregated state is very crucial for advance of delayed luminescence-based applications such as time-gated bio-sensing and temperature sensing. We report herein that synthesis, photophysical properties, molecular and crystal structures, and theoretical calculations of 2,6-bis (diarylamino)benzophenones. Absorption spectra in solution and calculations using density functional theory (DFT) method revealed that the optical excitation took place through intramolecular charge-transfer from one diarylamino moiety to an aroyl group. While the benzophenones did not luminesce in solution, the solids of the benzophenones emitted green light with moderate-to-good quantum yields. Thus, the benzophenones exhibit aggregation-induced emission. Based on the lifetime measurement, the green emission of the solids was found to include TADF. The emergence of the TADF is supported by the small energy gap between the excited singlet and triplet states, which was estimated by time-dependent DFT calculations. Thin films of poly(methyl methacrylate) doped by the benzophenones also showed green prompt and delayed fluorescence whose lifetimes were in the order of microseconds. Linear correlation between logarithm value of TADF lifetime and temperature was observed with the benzophenone in powder, suggesting that the benzophenones can serve as molecular thermometers workable under aqueous conditions.
Similar content being viewed by others
References
Uoyama H, Goushi K, Shizu K, Nomura H, Adachi C. Nature, 2012, 492: 234–238
Nakanotani H, Higuchi T, Furukawa T, Masui K, Morimoto K, Numata M, Tanaka H, Sagara Y, Yasuda T, Adachi C. Nat Commun, 2014, 5: 4016
Zhang Q, Li B, Huang S, Nomura H, Tanaka H, Adachi C. Nat Photon, 2014, 8: 326–332
Kaji H, Suzuki H, Fukushima T, Shizu K, Suzuki K, Kubo S, Komino T, Oiwa H, Suzuki F, Wakamiya A, Murata Y, Adachi C. Nat Commun, 2015, 6: 8476
Marriott G, Clegg RM, Arndt-Jovin DJ, Jovin TM. Biophys J, 1991, 60: 1374–1387
Carretero AS, Castillo AS, Gutiérrez AF. Crit Rev Anal Chem, 2005, 35: 3–14
Suhling K. French PMW, Phillips D. Photochem Photobiol Sci, 2005, 4: 13–22
Xiong X, Song F, Wang J, Zhang Y, Xue Y, Sun L, Jiang N, Gao P, Tian L, Peng X. J Am Chem Soc, 2014, 136: 9590–9597
Shimizu M, Hiyama T. Chem Asian J, 2010, 5: 1516–1531
Li Q, Li Z. Adv Sci, 2017, 4: 1600484
Wong MY, Zysman-Colman E. Adv Mater, 2017, 29: 1605444
Im Y, Kim M, Cho YJ, Seo JA, Yook KS, Lee JY. Chem Mater, 2017, 29: 1946–1963
Yang Z, Mao Z, Xie Z, Zhang Y, Liu S, Zhao J, Xu J, Chi Z, Aldred MP. Chem Soc Rev, 2017, 46: 915–1016
Tao Y, Yuan K, Chen T, Xu P, Li H, Chen R, Zheng C, Zhang L, Huang W. Adv Mater, 2014, 26: 7931–7958
Chen B, Sun X, Evans RE, Zhou R, Demas JN, Trindle CO, Zhang G. J Phys Chem A, 2015, 119: 8854–8859
Xu S, Liu T, Mu Y, Wang YF, Chi Z, Lo CC, Liu S, Zhang Y, Lien A, Xu J. Angew Chem Int Ed, 2015, 54: 874–878
Xie Z, Chen C, Xu S, Li J, Zhang Y, Liu S, Xu J, Chi Z. Angew Chem Int Ed, 2015, 54: 7181–7184
Gan S, Luo W, He B, Chen L, Nie H, Hu R, Qin A, Zhao Z, Tang BZ. J Mater Chem C, 2016, 4: 3705–3708
Furue R, Nishimoto T, Park IS, Lee J, Yasuda T. Angew Chem Int Ed, 2016, 55: 7171–7175
Tsujimoto H, Ha DG, Markopoulos G, Chae HS, Baldo MA, Swager TM. J Am Chem Soc, 2017, 139: 4894–4900
Guo J. Li X-L, Nie H, Luo W, Gan S, Hu S, Hu R, Qin A, Zhao Z, Su S-J, Tang BZ. Adv Funct Mater, 2017, 1606458
Wang T, Wu Z, Sun W, Jin S, Zhang X, Zhou C, Jiang J, Luo Y, Zhang G. J Phys Chem A, 2017, 121: 7183–7190
Guo J, Li XL, Nie H, Luo W, Hu R, Qin A, Zhao Z, Su SJ, Tang BZ. Chem Mater, 2017, 29: 3623–3631
Shimizu M, Takeda Y, Higashi M, Hiyama T. Angew Chem Int Ed, 2009, 48: 3653–3656
Shimizu M, Asai Y, Takeda Y, Yamatani A, Hiyama T. Tetrahedron Lett, 2011, 52: 4084–4089
Shimizu M, Takeda Y, Higashi M, Hiyama T. Chem Asian J, 2011, 6: 2536–2544
Shimizu M, Kaki R, Takeda Y, Hiyama T, Nagai N, Yamagishi H, Furutani H. Angew Chem Int Ed, 2012, 51: 4095–4099
Shimizu M, Tamagawa T. Eur. Org Chem, 2015, 2015: 291–295
Shimizu M, Fukui H, Natakani M, Sakaguchi H. Eur. Org Chem, 2016, 2016: 5950–5956
Shimizu M, Fukui H, Shigitani R. Jnl Chin Chem Soc, 2016, 63: 317–322
Shimizu M, Kimura A, Sakaguchi H. Eur. Org Chem, 2016, 2016: 467–473
Shimizu M, Shigitani R, Nakatani M, Kuwabara K, Miyake Y, Tajima K, Sakai H, Hasobe T. J Phys Chem C, 2016, 120: 11631–11639
Shimizu M, Kinoshita T, Shigitani R, Miyake Y, Tajima K. Mater Chem Front, 2018, 2: 347–354
Shimizu M, Nakatani M. Eur. Org Chem, 2017, 2017: 4695–4702
Mei J. Leung NLC, Kwok RTK, Lam JWY, Tang BZ. Chem Rev, 2015, 115: 11718–11940
Mei J, Hong Y. Lam JWY, Qin A, Tang Y, Tang BZ. Adv Mater, 2014, 26: 5429–5479
CCDC 1840378 (for 1b) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09, revision D. 01. Wallingford: Gaussian, Inc., 2013
Schrum KF, Williams AM, Haerther SA, Ben-Amotz D. Anal Chem, 1994, 66: 2788–2790
Fister JC, Rank D, Harris JM. Anal Chem, 1995, 67: 4269–4275
Uchiyama S. Prasanna de Silva A, Iwai K. J Chem Educ, 2006, 83: 720–727
Brites CDS, Lima PP, Silva NJO, Millán A, Amaral VS, Palacio F, Carlos LD. Nanoscale, 2012, 4: 4799–4829
McLaurin EJ, Bradshaw LR, Gamelin DR. Chem Mater, 2013, 25: 1283–1292
Bai T, Gu N. Small, 2016, 12: 4590–4610
Uchiyama S, Gota C, Tsuji T, Inada N. Chem Commun, 2017, 53: 10976–10992
Acknowledgements
This work was supported by Grants-in-Aid for JSPS KAKENHI (15H03795), MEXT KAKENHI 15K13671, the Nagase Science and Technology Foundation, and the Ogasawara Foundation for the Promotion of Science and Engineering.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shimizu, M., Nakatani, M. & Nishimura, K. Aggregation-induced emission and thermally activated delayed fluorescence of 2,6-diaminobenzophenones. Sci. China Chem. 61, 925–931 (2018). https://doi.org/10.1007/s11426-018-9301-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-018-9301-6