Abstract
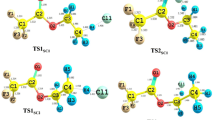
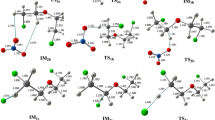
The mechanism, kinetics, and thermochemistry of the gas-phase reactions of CF2ClC(O)OCH2CH3,ethyl chlorodifluoroacetate (ECDFA) with the OH radical and Cl atom are investigated. Geometry optimization and frequency calculations have been performed at the MPWB1K/6-31+G(d,p) level of theory and energetic information is refined by using G2(MP2) theory. Transition states are searched on the potential energy surface of reaction channels and each of the transition states is characterized by the presence of only one imaginary frequency. Connections of the transition states between designated local minima are confirmed by intrinsic reaction coordinate calculation. Theoretically calculated rate constants at 298 K using the Canonical Transition State Theory are found to be in good agreement with the experimentally measured ones. Using group-balanced isodesmic reactions as working chemical reactions, the standard enthalpies of formation for CF2ClC(O)OCH2CH3, CF2ClC(O)OCH2CH2, and CF3C(O)OCHCH3 are also reported for the first time. The hydrogen abstraction occurs mainly from –CH2 group. The T1 diagnostic calculation suggests that the multi-reference character is not an issue for such systems. The estimated atmospheric life time of ECDFA is expected to be around 24 days.


Similar content being viewed by others
References
Tsai WT (2005) J Hazard Mater 119:69–78
Sekiya A, Misaki S (2000) J Fluor Chem 101:215–221
Ravishankara RA, Turnipseed AA, Jensen NR, Barone S, Mills M, Howark CJ, Solomon S (1994) Science 263:71–75
Oyaro N, Sellevag SR, Nielsen C (2005) J Phys Chem A 109:337–346
Urata S, Takada A, Uchimaru T, Chandra AK (2003) Chem Phys Lett 368:215–223
Dalmasso PR, Nieto JD, Taccone RA, Teruel MA, Lane SI (2006) J Phys Org Chem 19:771–775
Singh HJ, Mishra BK (2010) J Mol Model 16:1473–1480
Singh HJ, Mishra BK (2011) J Mol Model 17:415–422
Chandra AK (2012) J Mol Model 18:4239–4247
Chen L, Kutsuna S, Tokuhashi K, Sekiya A (2004) Chem Phys Lett 400:563–568
Singh HJ, Mishra BK, Rao PK (2010) Bull Korean Chem Soc 31:3718–3722
Blanco MB, Barnes I, Teruel MA (2010) J Phys Org Chem 23:950–954
Ninomiya Y, Kawasaki M, Guschin A, Molina LT, Molina MJ, Wallington TJ (2000) Environ Sci Technol 34(14):2973–2978
Dalmasso PR, Taccone RA, Nieto JD, Teruel MA, Lane SI (2006) Atmos Environ 40:7298–7303
Wingenter OW, Kubo MK, Blake NJ, Smith TW, Blake DR (1996) J Geophys Res 101:4331–4340
Andersen MPS, Nielsen OJ, Wallington TJ, Hurley MD, DeMoore GW (2005) J Phys Chem A 109:3926–3934
Mellouki A, Bras GL, Sidebottom H (2003) Chem Rev 103:5077–5096
Blanco MB, Teruel MA (2007) Chem Phys Lett 441:1–6
Blanco MB, Teruel MA (2007) Atmos Environ 41(34):7330–7338
Blanco MB, Bejan I, Barnes I, Wiesen P, Teruel MA (2008) Chem Phys Lett 453:18–23
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr., Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell K, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09, revision B.01. Gaussian Inc., Wallingford
Zhao Y, Truhlar DG (2004) J Phys Chem A 108:6908–6918
Chakrabatty AK, Mishra BK, Bhattacharjee D, Deka RC (2013) Mol Phys 111:860–867
Mishra BK, Chakrabatty AK, Deka RC (2013) J Mol Model 19:2189–2195
Mishra BK, Chakrabatty AK, Bhattacharjee D, Deka RC (2013) Struct Chem (in press). doi:10.1007/s11224-013-0203-7
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154–2161
Curtiss LA, Raghavachari K, Pople JA (1993) J Chem Phys 98:1293–1298
Alecu IM, Zheng J, Zhao Y, Truhlar DG (2010) J Chem Theor Comput 6:2872–2887
Rienstra-Kiracofe JC, Allen WD, Schaefer HF III (2000) J PhysChem A 104:9823–9840
Lee TJ, Taylor PR (1989) Int J Quant Chem Quant Chem Symp S23:199–207
Kuchitsu K (1998) Structure of free polyatomic molecules basic data, vol 1. Springer, Berlin, p 58
Zhurko G, Zhurko D (2011) ChemCraft Program Academic, version 1.6
Hammond GS (1955) J Am Chem Soc 77:334–338
Lide DR (2008–2009) CRC handbook of chemistry and physics, 89th edn. CRC Press, New York
Good DA, Fransisco JS (1998) J Phys Chem A 102:7143–7148
Chase MW Jr (1998) JANAF Thermochemical tables, 3th edn. J Phys Chem Ref Data Monogr 9:1–1951
Manion JA (2002) J Phys Chem Ref Data 31:123–172
Wiberg KB, Crocker LS, Morgan KM (1991) J Am Chem Soc 113:3447–3450
Pilcher G, Pell AS, Coleman D (1964) J Trans Faraday Soc 60:499–505
Csontos J, Rolok Z, Das S, Kallay M (2010) J Phys Chem A 114:13093–13103
Laidler KJ (2004) Chemical kinetics, 3rd edn. Pearson Education, New Delhi
Wigner EP (1932) Z Phys Chem B19:203–216
Truhlar DG, Chuang YY (2000) J Chem Phys 112:1221–1228
Mao WX, Long ZW, Wang YB, Long CY, Qin SJ (2013) Struct Chem 24:383–392
Andersen VF, Berhanu TA, Nilsson EJK, Jørgensen S, Nielsen OJ, Wallington TJ, Johnson MS (2011) J Phys Chem A 115:8906–8919
Jørgensen S, Andersen VF, Nilsson EJK, Nielsen OJ, Berhanu TA, Johnson MS (2010) Chem Phys Lett 490:116–122
Singh HJ, Mishra BK, Gour NK (2010) Theor Chem Acc 125:57–64
Singh HJ, Mishra BK, Gour NK (2009) Bull Korean Chem Soc 30:2973–2978
Chandra AK, Uchimaru T (2000) J Phys Chem A 104:8535–8539
Papadimitriou VC, Kambanis KG, Lazarou YG, Papagiannakopoulos P (2004) J Phys Chem A 108:2666–2674
Kurylo MJ, Orkin VL (2003) Chem Rev 103:5049–5076
Spicer CW, Chapman EG, Finlayson-Pitts BJ, Plastridge RA, Hubbe JM, Fast JD, Berkowitz CM (1998) Nature 394:353–356
Acknowledgments
The authors are thankful to Department of Science and Technology, New Delhi for financial support. BKM thanks University Grants Commission, New Delhi for providing post doctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mishra, B.K., Chakrabartty, A.K. & Deka, R.C. A theoretical investigation on the kinetics and reactivity of the gas-phase reactions of ethyl chlorodifluoroacetate with OH radical and Cl atom at 298 K. Struct Chem 25, 463–470 (2014). https://doi.org/10.1007/s11224-013-0312-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-013-0312-3