Abstract
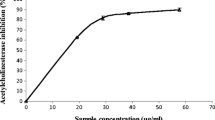
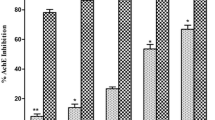
Inhibitory effects of a fresh leaf juice of the Withania somnifera (W.s.) on the enzymes acetylcholinesterase (AChE; EC 3.1.17) and nicotinamide adenine dinucleotide diaphorase (NADPH-d) were studied in the mice brain using histochemical and biochemical approaches. The results obtained show significant reduction in the number of and reaction intensity in AChE- and NADPH-d-positive neurons within selected areas of the brains of W.s.-treated mice. Colocalization of AChE and NADPH-d activity also showed significant reduction in the number of cells positive for both enzymes after W.s. treatment, as compared to cells with isolated localization of AChE or NADPH-d. Biochemical estimation of the AChE and NADPH-d levels in brain tissue also showed significant dose-dependent inhibition of the activity of both enzymes after W.s. treatment. In conclusion, our study allows us to suggest that W.s. constituents significantly inhibit both AChE and NADPH-d activity. Inhibition of AChE is direct, while NADPH-d is inhibited indirectly. This study might be important for possible therapeutic use of W.s. in neurodegenerative disorders, in particular in Alzheimer’s disease where inhibition of both AChE and nNOS is important.
Similar content being viewed by others
References
M. A. Weiner and J. Weiner, “Ashwagandha (Indian ginseng),” in: Herbs that Heal, Quantum Books, Mill Valley (1994), pp. 70–72.
A. V. Muruganandam, V. Kumar, and S. K. Bhattacharya, “Effect of polyherbal formulation, EuMil, on chronic stress-induced homeostatic perturbations in rats,” Indian J. Exp. Biol., 40, No. 10, 1151–1160 (2002).
S. K. Bhattacharya and A. V. Muruganandam, “Adaptogenic activity of Withania somnifera: an experimental study using a rat model of chronic stress,” Pharmacol. Biochem. Behav., 75, No. 3, 547–555 (2003).
B. Singh, B. K. Chandan, and D. K. Gupta, “Adaptogenic activity of a novel withanolide-free aqueous fraction from the roots of Withania somnifera Dun. [Part II],” Phytother. Res., 17, No. 5, 531–536 (2003).
V. N. Dadkar, N. U. Ranadive, and H. L. Dhar, “Evaluation of antistress (adaptogen) activity of Withania somnifera (Ashwagandha),” Indian J. Clin. Biochem., 2, 101–108 (1987).
R. Schliebs, A. Liebmann, S. K. Bhattacharya, et al., “Administration of defined extracts from Withania somnifera (Indian ginseng) and Shilajit differentially affects cholinergic but not glutamatergic and GABAergic markers in rat brain,” Neurochem. Int., 30, No. 2, 181–190 (1997).
J. B. Melo, P. Agostinho, and C. R. Oliveira, “Involvement of oxidative stress in the enhancement of acetylcholinesterase activity induced by amyloid beta peptide,” Neurosci. Res., 45, 117–127 (2003).
J. E. Brenman and D. S. Bredt, “Synaptic signaling by nitric oxide,” Current Opin. Neurobiol., 7, 374–378 (1997).
E. M. Schuman and D. V. Madison, “Nitric oxide and synaptic function,” Ann. Rev. Neurosci., 17, 153–157 (1994).
Y. Yin, L. Hung, H. Liu, et al., “Effect of tanshinone on the levels of nitric oxide synthase and acetylcholinesterase in the brain of Alzheimer’s disease rat model,” Clin. Invest. Med., 31, E248-E257 (2008).
P. J. Houghton and M. J. Howes, “Natural products and derivatives affecting neurotransmission relevant to Alzheimer’s and Parkinson’s disease,” Neurosignals, 14, 6–22 (2005).
M. Bhatnagar, D. Sharma, and M. Salvi, “Neuroprotective effects of Withania somnifera Dunal: A possible mechanism,” Neurochem. Res., 34, 1975–1983 (2009).
D. S. Bredt, C. E. Glatt, and P. M. Hwang, “Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase,” Neuron, 7, No. 4, 615–624 (1991).
D. S. Bredt and H. S. Snyder, “Nitric oxide, a novel neuronal messenger,” Neuron, 8, 3–11 (1992).
S. R. Vincent and H. Kimura, “Histochemical mapping of nitric oxide synthase in rat brain,” Neuroscience, 46, 755–784 (1992).
M. J. Karnovasky and L. Roots, “A ‘direct-coloring’ thiocholine method for cholinesterase,” J. Histochem. Cytochem., 12, 2–19 (1964).
B. T. Hope and S. R. Vincent, “Histochemical characterization of neuronal NADPH-diaphorase,” J. Histochem. Cytochem., 37, 653–661 (1989).
G. Paxinos and K. B. J. Franklin, “The mouse brain in stereotaxic coordinates,” Acad. Press, San Diego (2001).
G. L. Ellman, K. D. Courtney, V. Andres Jr., and R. M. Featherstone, “A new and rapid colorimetric determination of acetylcholinesterase activity,” Biochem. Pharmacol., 7, No. 2, 88–95 (1961).
B. A. Weissman and S. S. Gross, “Measurement of NO and NO synthase,” Current Protoc. Neurosci., Chap. 7, Unit 7.13 (2001), Published online: 1 May, 2001; doi: 10.1002/0471142301. ns0713s05.
Sunanda, B. S. Rao, and T. R. Raju, “Corticosterone attenuates zinc-induced neurotoxicity in primary hippocampal culture,” Brain Res., 791, Nos. 1/2, 295–298 (1998).
V. A. Talesa, “Acetylcholinesterase in Alzheimer’s disease,” Mech. Ageing Dev., 12, 1961–1969 (2001).
E. Giacobini, “Cholinergic system in Alzheimer’s disease,” Prog. Brain Res., 84, 321–322 (1990).
J. Ulrich, W. Meier-Ruge, A. Probst, et al., “Senile plaques: staining for acetylcholinesterase and A4 protein: a comparative study in the hippocampus and entorhinal cortex,” Acta. Neuropathol., 80, 624–628 (1990).
M. A. Morán, E. J. Mufson, and P. J. Gómez-Ramos, “Colocalization of cholinesterases with beta amyloid protein in aged and Alzheimer’s brains,” Acta. Neuropathol., 85, 362–369 (1993).
B. Vinutha, H. D. Prasantha, K. Salma, et al., “Screening of selected Indian medicinal plants for acetylcholinesterase inhibitory activity,” Ethanopharmacology, 109, 359–363 (2007).
M. T. Chowdhry, S. Yousuf, S. A. Nawajz, et al., “Cholinesterase inhibiting withanolides from Withania somnifera,” Chem. Pharm. Bull., 52, 1358–1361 (2004).
H. J. Luth, N. Holzer, U. Gartner, et al., “Expression of endothelial and inducible NOS isoforms is increased in Alzheimer’s disease in APP23 transgenic mice and after experimental brain lesion in rat: evidence for an induction by amyloid pathology,” Brain Res., 913, No. 1, 57–67 (2001).
D. Kluchova, K. Schmidtova, S. Rybarova, et al., “Partial colocalization of NADPH-diphorase and acetylcholinesterase positivity in spinal cord neurons,” Physiol. Res., 49, No. 1, 151–155 (2000).
A. Law, S. Gauthier, and R. Quirion, “Say NO to Alzheimer’s disease: putative links between nitric oxide and dementia of Alzheimer’s type,” Brain Res. Rev., 1, 73–96 (2001).
A. Law, S. Gauthier, and R. Quirion, “ Neuroprotective and neurorescuing effects of isoform-specific nitric oxide synthase inhibitors, nitric oxide scavengers, and antioxidant against beta-amyloid toxicity,” Br. J. Pharmacol., 133, No. 7, 1114–1124 (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhatnagar, M., Suhalka, P., Sukhwal, P. et al. Inhibition of Acetylcholinesterase and NO Synthase Activity in the Mice Brain: Effect of a Withania Somnifera Leaf Juice. Neurophysiology 44, 301–308 (2012). https://doi.org/10.1007/s11062-012-9300-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11062-012-9300-2