Abstract
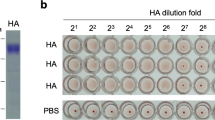
Influenza A viruses are subtyped according to antigen characterization of hemagglutinin (HA) and neuraminidase surface glycoproteins. The hemagglutination inhibition (HI) assay using reference antiserum is currently applied to serologic screening of subtype-specific antibodies in sera. The reference antiserum is made by injecting chickens with live or inactivated whole virus preparations. Nonspecific inhibitors of antisera prepared by the conventional method may affect the specificity of HI assay. In this study, highly pure recombinant proteins generated using baculovirus expression vector system based on full-length of HA (HAF) and antigenic region of HA1 genes of H9 subtype, and also inactivated whole virus were used to immunization of chickens. Measurable antibody titers were present for treated birds after 3 weeks and generally increased after each boost. The performance of the prepared antisera was evaluated by testing a panel of known standard strains of influenza virus representing five HA subtypes. Relative to the conventional method using whole virus immunization and recombinant HAF protein, the antiserum prepared by recombinant HA1 had a specificity of 100% for all tested subtypes. The antiserum prepared by expression of HA1 protein in baculovirus has the potential for rapid and specific HA subtyping of influenza viruses without producing antibodies specific to other viral proteins.



Similar content being viewed by others
References
Fouchier RA, Munster V, Wallensten A (2005) Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J Virol 79:2814–2822
Butt KM, Smith GJD, Chen H, Zhang LJ, Connie Leung YH, Xu KM, Lim W, Webster RG, Yuen KY, Malik Peiris JS, Guan Y (2005) Human infection with an avian H9N2 influenza A virus in Hong Kong in 2003. J Clin Microbiol 43:5760–5767
Guan Y, Poon LLM, Cheung CY, Ellis TM, Lim W, Lipatov AS, Chan KH, Peiris JSM (2004) H5N1 influenza: a protean pandemic threat. PNAS 101:8156–8161
Shaw M, Cooper L, Xu X, Thompson W, Krauss S, Guan Y, Zhou N, Subbarao K (2002) Molecular changes associated with the transmission of avian influenza A H5N1 and H9N2 viruses to humans. J Med Virol 66:107–114
Guan Y, Shortridge KF, Krauss S, Chin PS, Dyrting KC, Ellis TM, Webster RG, Peiris M (2000) H9N2 influenza viruses possessing H5N1-like internal genomes continue to circulate in Southeastern China. J Virol 74:9372–9380
Guan Y, Shortridge KF, Krauss S, Webster RG (1999) Molecular characterization of H9N2 influenza viruses: were they the donors of the internal genes of H5N1 viruses in Hong Kong? PNAS 96:9363–9367
Peiris M, Yam WC, Chan KH, Ghose P, Shortridge KF (1999) Influenza A H9N2: aspects of laboratory diagnosis. J Clin Microbiol 37:3426–3427
Swayne DE, Senne DA, Beard CW (1998) Influenza. In: Isolation and identification of avian pathogens, 4th edn. American association of avian Pathologists, University of Pennsylvania, USA, pp 235–240
Phipps LP, Essen SC, Brown IH (2004) Genetic subtyping of influenza A viruses using RT-PCR with a single set of primers based on conserved sequences within the HA2 coding region. J Virol Methods 122:119–122
Lee CW, Suarez DL (2004) Application of real-time RT-PCR for the quantitation and competitive replication study of H5 and H7 subtype avian influenza virus. J Virol Methods 119:151–158
Mori Y, Notomi T (2009) Loop-mediated isothermal amplification (LAMP): a rapid, accurate, and cost-effective diagnostic method for infectious diseases. J Infect Chem 15:62–69
Ong WT, Omar AR, Ideris A, Seyed Hassan S (2007) Development of a multiplex real-time PCR assay using SYBR Green 1 chemistry for simultaneous detection and subtyping of H9N2 influenza virus type A. J Virol Methods 144:57–64
Office International des Epizooties (2005) Avian influenza. In: Manual of diagnostic tests and vaccines for terrestrial animals, 5th edn. Office International des Epizooties, Paris
Lee C-W, Senne DA, Suarez DL (2006) Development and application of reference antisera against 15 hemagglutinin subtypes of influenza virus by DNA vaccination of chickens. Clin Vaccine Immunol 13:395–402
Bac-to-Bac Baculovirus Expression System 2002. version C. Invitrogen life technologies
Okamatsu M, Sakoda Y, Kishida N, Isoda N, Kida H (2008) Antigenic structure of the hemagglutinin of H9N2 influenza viruses. Arch Virol 153:2189–2195
Gambaryan A, Yamnikova S, Lvov D, Tuzikov A, Chinarev A et al (2005) Receptor specificity of influenza viruses from birds and mammals: new data on involvement of the inner fragments of the carbohydrate chain. Virology 334:276–283
Skehel JJ, Wiley DC (2000) Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem 69:531–569
Kost T, Condreay PJ, Jarvis DL (2005) Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol 23:567–575
Jones T, Allard F, Cyr SL, Tran SP, Plante M, Gauthier J, Bellerose N, Lowell GH, Burt DS (2003) A nasal proteosome influenza vaccine containing baculovirus-derived hemagglutinin induces protective mucosal and systemic immunity. Vaccine 21:3706–3712
Spackman E, Senne DA, Myers TJ, Bulaga LL, Garber LP, Perdue ML, Lohman K, Suarez DL (2002) Development of a real-time RT-PCR assay type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J Clin Microbiol 40:3250–3256
Bosch FX, Garten W, Klenk HD, Rott R (1981) Proteolytic cleavage of influenza virus hemagglutinins: primary structure of the connecting peptide between HA1 and HA2 determines proteolytic cleavability and pathogenicity of avian influenza virus. Virology 113:725–735
Graves PN, Schulman JL, Young JF, Palese P (1983) Preparation of influenza virus subviral particles lacking the HA1 subunit of hemagglutinin: unmasking of cross-reactive HA2 determinants. Virology 126:106–116
Gerhard W (2001) The role of the antibody response in influenza virus infection. Curr Top Microbiol Immunol 260:171–190
Cox RJ, Brokstad KA (1999) The postvaccination antibody response to influenza virus proteins. APMIS 107:289–296
Gocník M, Fislová T, Mucha V, Sládková T, Russ G, Kostolanský F, Varečková E (2008) Antibodies induced by the HA2 glycopolypeptide of influenza virus hemagglutinin improve recovery from influenza A virus infection. J Gen Virol 89:958–967
Vare-kovfi E, Mueha V, Betfikovfi T, Russ G (1993) Monoclonal antibodies demonstrate accessible HA2 epitopes in minor subpopulation of native influenza virus hemagglutinin molecules. Arch Virol 130:45–56
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shahsavandi, S., Salmanian, AH., Ghorashi, S.A. et al. Specific subtyping of influenza A virus using a recombinant hemagglutinin protein expressed in baculovirus. Mol Biol Rep 38, 3293–3298 (2011). https://doi.org/10.1007/s11033-010-0434-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0434-2