Abstract
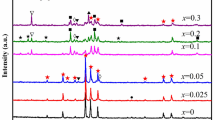
This contribution is devoted to thermoanalytical investigation of the mechanism of the formation of solid solutions of Li1+xCrxZr2−x(PO4)3. The samples were prepared by high-temperature solid-state reaction in the reaction mixtures of (0.5 + x/2)Li2CO3–(2 − x)ZrOCl2·8H2O–(x/2)Cr2O3–3(NH4)2HPO4. Thermal behaviour of the mixtures was characterised using STA analysis (RT-1200 °C); evolution of the phase composition during heating was analysed using powder XRD analysis. It is shown that with increase in substitution degree x from 0 to 2, the mechanism of the formation of the solid solutions changes dramatically. In general, it is influenced by the content of ZrOCl2·8H2O because its interaction with (NH4)2HPO4 is the dominant feature of the thermal transformation of the mixtures. However, with decrease in ZrOCl2·8H2O content and the corresponding increase in x, the indicated process becomes less dominant. On the other hand, unreacted amount of (NH4)2HPO4 is increased and the corresponding effect of elimination of its ammonia becomes more and more prominent. The mixture with x = 2 can be characterised with typical behaviour of the mixtures of (NH4)2HPO4 with oxides or carbonates. In general, the formation of solid solutions required calcination at 1200 °C during 6–12 h.


Similar content being viewed by others
References
Gorodylova N, Kosinová V, Šulcová P, Bělina P, Vlček M. Cr1/3Zr2P3O12 with unusual tetrahedral coordination of Cr(III): peculiarities of the formation, thermal stability and application as a pigment. Dalton Trans. 2014;43(41):15439–49.
Gorodylova N, Kosinová V, Dohnalová Ž, Bělina P, Šulcová P. New purple-blue ceramic pigments based on CoZr4(PO4)6. Dyes Pigm. 2013;98:393–404.
Oikonomou P, Dedeloudis Ch, Stournaras CJ, Ftikos C. [NZP]: a new family of ceramics with low thermal expansion and tunable properties. J Eur Ceram Soc. 2007;27:1253–6.
Zhang Y, Chen K, Shen Y, Lin Y, Nan C-W. Enhanced lithium-ion conductivity in a LiZr2(PO4)3 solid electrolyte by Al doping. Ceram Int. 2017; ahead of print.
Mutter D, Urban DF, Elsaesser Ch. Systematic search for lithium ion conducting compounds by screening of compositions combined with atomistic simulation. MRS Adv. 2017;2(9):483–9.
Orlova AI. Isomorphism in d-and f-element phosphates having framework crystal structure and crystallochemical conception of NZP matrix for radionuclide immobilisation. Czech J Phys. 2003;53A:649–55.
Wang T, Yu Q, Kong J, Wong Ch. Synthesis and heat-insulating properties of yttria-stabilized ZrO2 hollow fibers derived from a ceiba template. Ceram Int. 2017;43(12):9296–302.
Xiao L, Xu L-F, Hua K-H, Shui A-Z. Preparation of Al2O3–ZrO2 composite nanopowders by co-precipitation method. J Synth Cryst. 2015;44(10):2751–5.
Thananatthanachon T. Synthesis and characterization of a perovskite barium zirconate (BaZrO3): an experiment for an advanced inorganic chemistry laboratory. J Chem Educ. 2016;93(6):1120–3.
Zhao D, Deng X, Ding Z, Wang H, Ma G. Intermediate temperature ionic conduction in Mg2+-doped ZrP2O7 ceramics. Solid State Ion. 2012;229:33–7.
Gorodylova N, Šulcová P, Bosacka M, Filipek E, Vlček M. Heterovalent Zr4+–Cu2+ substitution in zirconium pyrophosphate: from theoretical models to synthesis and utilisation. J Eur Ceram Soc. 2015;35:4293–305.
Tao X, Xiang Zh, Zhou Sh, Zhu Y, Qiu W. Synthesis of a soluble preceramic polymer for ZrC using 2-hydroxybenzyl alcohol as carbon source. Adv Appl Ceram. 2016;115(6):342–8.
Liu Y, Geng R, Cui Y, Peng S, Chang X, Han K, Yu M. A novel liquid hybrid precursor method via sol-gel for the preparation of ZrB2 films. Mater Des. 2017;128:80–5.
Patra N, Nasiri NA, Jayaseelan DD, Lee WE. Synthesis, characterization and use of synthesized fine zirconium diboride as an additive for densification of commercial zirconium diboride powder. Ceram Int. 2016;42(8):9565–70.
Guo Ch. Preparation and applications of kaolin ZrO2/ZnO loaded photo-catalytic functional materials. Mater Res Appl. 2015;9(3):162–5.
Bhattacharyya KG, Sen Gupta S. Adsorption of Fe(III), Co(II) and Ni(II) on ZrO-kaolinite and ZrO-montmorillonite surfaces in aqueous medium. Colloids Surf A Physicochem Eng Asp. 2008;317(1–3):71–9.
Nikoofar K, Khademi Z. A review on green Lewis acids: zirconium(IV) oxydichloride octahydrate (ZrOCl2·8H2O) and zirconium(IV) tetrachloride (ZrCl4) in organic chemistry. Res Chem Intermed. 2016;42(5):3929–77.
Gorodylova N, Šulcová P, Bosacka M, Filipek E. DTA–TG and XRD study on the reaction between ZrOCl2·8H2O and (NH4)2HPO4 for synthesis of ZrP2O7. J Therm Anal Calorim. 2014;2014(118):1095–100.
Gorodylova N, Kosinová V, Šulcová P. Interrelations between composition, structure, thermal stability, and chromatic characteristics of new NASICON-related solid solutions of Li1+x Cr x Zr2−x (PO4)3. Ceram Int. 2017;. doi:10.1016/j.ceramint.2017.07.135.
Kim JW, Lee HG. Thermal and carbothermic decomposition of Na2CO3 and Li2CO3. Metallurg Mater Trans B. 2001;32:17–24.
Stenina IA, Velikodnyi YuA, Ketsko VA, Yaroslavtsev AB. Synthesis of NASICON-type lithium zirconium phosphate. Inorg Mater. 2004;40:967–70.
Acknowledgements
The authors would like to thank for the financial support to Grant Agency of Czech Republic (No. 16-06697S).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gorodylova, N., Šulcová, P. DTA–TGA and XRD study of the formation of LISICON-type Li1+xCrxZr2−x(PO4)3 ceramic using ZrOCl2·8H2O as precursor. J Therm Anal Calorim 133, 405–411 (2018). https://doi.org/10.1007/s10973-017-6736-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6736-z