Abstract
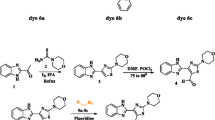
Novel carbazole based styryl derivatives (6a–6c) having styryl group at third position and a methoxy substitution were synthesized by condensing 4-methoxy-9-methyl-9H-carbazole-3-carbaldehyde 3 and different active methylene derivatives (5a–5c). Evaluated photophysical properties of these synthesized novel chromophores, studied the effect of solvent polarity on absorption, emission and quantum yield of these styryl derivatives. DFT and TD-DFT computations are carried out to study structural, molecular, electronic and photophysical parameters of dyes. The ratio of ground state to excited state dipole moment was calculated using Bakhshiev and Kawski-Chamma-Viallet correlations.










Similar content being viewed by others

References
Tsai M-H, Ke T-H, Lin H-W, Chung-Chih W, Chiu S-F, Fang F-C, Liao Y-L, Wong K-T, Chen Y-H, Wu C-I (2009) Triphenylsilyl- and trityl-substituted carbazole-based host materials for blue electrophosphorescence. ACS Appl Mater Interfaces 1(3):567–574
Justin Thomas KR, Lin JT, Tao Y, Ko C (2001) Light-emitting carbazole derivatives: potential electroluminescent materials. J Am Chem Soc 123:9404–9411
Li Y, Ding J, Day M, Tao Y, Lu J, D’iorio M (2003) Novel stable blue-light-emitting oligofluorene networks immobilized by boronic acid anhydride linkages. Chem Mater 15:4936–4943
Li J, Li Q, Liu D (2011) Novel thieno-[3,4-b]-pyrazines cored dendrimers with carbazole dendrons: design, synthesis, and application in solution-processed red organic light-emitting diodes. Appl Mater Interfaces 3:2099–2107
Zhang Y, Wada T, Wang L, Sasabe H (1997) Amorphous conjugated carbazole trimers for photorefractive materials. Chem Mater 9:2798–2804
Boudreault PT, Wakim S, Blouin N, Simard M, Tessier C, Tao Y, Leclerc M (2007) Synthesis, characterization, and application of indolo[3,2-b]carbazole semiconductors. J Am Chem Soc 129:9125–9136
Wang E, Hou L, Wang Z, Ma Z, Hellstrom S, Zhuang W, Zhang F, Inganas O, Andersson MR (2011) Side-chain architectures of 2,7-carbazole and quinoxaline-based polymers for efficient polymer solar cells. Macromolecules 44:2067–2073
Baldo MA, Lamansky S, Burrows PE, Thompson ME, Forrest SR (1999) Very high-efficiency green organic light-emitting devices based on electrophosphorescence. Appl Phys Lett 75:4–6
Lamansky S, Djurovich PI, Abdel-Razzaq F, Garon S, Murphy DL, Thompson ME (2002) Cyclometalated Ir complexes in polymer organic light-emitting devices. J Appl Phys 92:1570–1575
Iraqi A, Wataru I (2004) Preparation and properties of 2,7-linked N-alkyl-9H-carbazole main-chain polymers. Chem Mater 16:442–448
Ponnapati R, Felipe MJ, Muthalagu V, Puno K, Wolff B, Advincula R (2012) Conjugated polymer network films of poly(p-phenylene vinylene) with hole-transporting carbazole pendants: dual photoluminescence and electrochromic behavior. Appl Mater Interfaces 4:1211–1218
Gupta VD, Padalkar VS, Phatangare KR, Patil VS, Umape PG, Sekar N (2011) The synthesis and photo-physical properties of extended styryl fluorescent derivatives of N-ethyl carbazole. Dyes Pigments 88:378–384
Weber L, Halama J, Werner V, Hanke K, Bohling L, Chrostowska A, Dargelos A, Maciejczyk M, Raza A, Stammler H, Neumann B (2013) 1,3,2-benzodiazaboroles with 1,3-pentafluorophenyl and tetrafluoropyridyl substituents as building blocks in luminescent compounds. Eur J Inorg Chem 24:4268–4279
Takagi K, Takao H, Nakagawa T (2010) Synthesis and characterization of nitrogen-linked carbazole-containing fluorescent polymers. J Polym Sci Polym Chem 48:3729–3735
Zhang Y, Wada T, Sasabe H (1996) Synthesis and characterization of second-order nonlinear optical main-chain polyurethanes containing acceptor-substituted carbazole units. Macromol Chem Phys 197:1877–1888
Li L, Wu Y, Zhou Q, He C (2012) Experimental and theoretical studies on the one-photon and two-photon properties of a series of carbazole derivatives containing styrene. J Phys Org Chem 25:362–372
Gupta VD, Padalkar VS, Phatangare KR, Patil VS, Umape PG, Sekar N (2011) The synthesis and photo-physical properties of extended styryl fluorescent derivatives of N-ethyl carbazole. Dyes Pigments 88(3):378–384
Mingzhu L, Zhu Y, Ma K, Cao L, Wang K (2012) Facile synthesis and photo-physical properties of cyano-substituted styryl derivatives based on carbazole/phenothiazine. Spectrochim Acta A Mol Biomol Spectrosc 95:128–134
Liang M, Zhong-Yuan Wang L, Zhang H-YH, Sun Z, Xue S (2011) New organic photosensitizers incorporating carbazole and dimethylarylamine moieties for dye-sensitized solar cells. Renew Energy 36(10):2711–2716
Kim D, Lee JK, Kang SO, Ko J (2007) Molecular engineering of organic dyes containing N-aryl carbazole moiety for solar cell. Tetrahedron 63(9):1913–1922
Grigoras M, Vacareanu L, Ivan T, Catargiu AM (2013) Photophysical properties of isoelectronic oligomers with vinylene, imine, azine and ethylene spacers bearing triphenylamine and carbazole end-groups. Dyes Pigments 98(1):71–81
Hu ZJ, Sun PP, Li L, Tian YP, Yang JX, Wu JY, Zhou HP, Tao LM, Wan CK, Li M, Chen GH, Tang HH, Tao XT, Jiang MH (2009) Two novel π-conjugated carbazole derivatives with blue two-photon-excited fluorescence. Chem Phys 355:91–98
Gao YH, Wu JY, Li YM (2009) A sulfur-terminal Zn(II) complex and its two-photon microscopy biological imaging application. J Am Chem Soc 131:5208–5213
Chen C, Lin JT, Yeh MP (2006) Stilbene like carbazole dimer-based electroluminescent materials. Tetrahedron 62:8564–8570
Zeng H, Wang G, Zeng G, Li J (2009) The synthesis, characterization and electroluminescent properties of zinc(II) complexes for single-layer organic light-emitting diodes. Dyes Pigments 83:155–161
Kajiyama S, Uemura Y, Miura H, Hara K, Koumura N (2012) Organic dyes with oligo-n-hexylthiophene for dye-sensitized solar cells: relation between chemical structure of donor and photovoltaic performance. Dyes Pigments 92:1250–1256
Diaz JL, Dobarro A, Vilacampa B, Velasco D (2001) Structure and optical properties of 2,3,7,9-polysubstituted carbazole derivatives. Experimental and theoretical studies. Chem Mater 13:2528–2536
Treutler O, Ahlrichs R (1995) Efficient molecular numerical integration schemes. J Chem Phys 102:346–354
Becke AD (1993) Structure and optical properties of 2,3,7,9-polysubstituted carbazole derivatives. Experimental and theoretical studies new mixing of Hartree-Fock and local density-functional theories. J Chem Phys 98:1372–1377
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Kim CH, Park J, Seo J, Park SJ, Joo T (2010) Excited state intramolecular proton transfer and charge transfer dynamics of a 2-(2′-hydroxyphenyl)benzoxazole derivative in solution. J Phys Chem A 114:5618–5629
Santra M, Moon H, Park MH, Lee TW, Kim Y, Ahn KH (2012) Dramatic substituent effects on the photoluminescence of boron complexes of 2-(Benzothiazol-2-yl)phenols. Chem Eur J 18:9886–9893
Li H, Niu L, Xu X, Zhang S, Gao F (2011) A comprehensive therotical investigation of intramolecular proton transfer in the excited states for some newly-designed diphenylethylene derivatives bearing 2-(2-hydroxy-phenyl)-benzotriazole part. J Fluoresc 21:1721–1728
Hehre WJ, Radom L, Schleyer PVR, Pople J (1986) Ab initio molecular orbital theory. Wiley, New York
Bauernschmitt R, Ahlrichs R (1996) Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem Phys Lett 256:454–464
Furche F, Rappaport D (2005) Density functional theory for excited states: equilibrium structure and electronic spectra. In: Olivucci M (ed) Computational photochemistry. Elsevier, Amsterdam, Vol 16: Chapter 3
Lakowicz JR (1999) Principles of fluorescence spectroscopy, 2nd edn. Kluwer, New York
Valeur B (2001) Molecular fluorescence: principles and applications. Wiley-VCH Verlag, Weinheim
Phatangare KR, Gupta VD, Tathe AB, Padalkar VS, Patil VS, Ramasami P, Sekar N (2013) ESIPT inspired fluorescent 2-(4-benzo[d]oxazol-2-yl)naphtho[1,2-d]oxazol-2-yl)phenol: experimental and DFT based approach to photophysical properties. Tetrahedron 69:1767–1777
Padalkar VS, Ramasami P, Sekar N (2013) A combined experimental and DFT-TDDFT study of the excited-state intramolecular proton transfer (ESIPT) of 2-(2′-hydroxyphenyl) imidazole derivatives. J Fluoresc 23(5):839–851
Gupta VD, Tathe AB, Padalkar VS, Patil VS, Phatangare KR, Umape PG, Ramasami P, Sekar N (2013) TDDFT investigation of the electronic structures and photophysical properties of fluorescent extended styryl push-pull chromophores containing carbazole unit. J Fluoresc 23(6):1121–1138
Padalkar VS, Ramasami P, Sekar N (2013) TD-DFT study of excited-state intramolecular proton transfer (ESIPT) of 2-(1,3-benzothiazol-2-yl)-5-(N, N-diethylamino)phenol with benzoxazole and benzimidazole analogues. Procedia Comput Sci 18:797–805
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09, revision C.01. Gaussian, Inc., Wallingford
Kawski A (1966) Push–pull (thio)barbituric acid derivatives in dye photosensitized radical and cationic polymerization reactions under 457/473 nm laser beams or blue LEDs. Acta Phys Polon 29:507–518
Milian B, Ort E, Hernandez V, Lopez Navarrete JT, Otsubo T (2003) Spectroscopic and theoretical study of push-pull chromophores containing thiophene-based quinonoid structures as electron spacers. J Phys Chem B 107:12175–12183
Kato S-i, Milan Kivala W, Schweizer B, Boudon C, Gisselbrecht J-P, Diederich F (2009) Origin of intense intramolecular charge-transfer interactions in nonplanar push–pull chromophores. Chem Eur J 15(35):8687–8691
Bakhshiev NG (1964) Universal intermolecular interactions and their effect on the position of the electronic spectra of molecules in two component solutions. Opt Spektrosk 16:821–832
Chamma A, Viallet PCR (1970) Determination du moment dipolaire d’une molecule dans un etat excite singulet. Acad Sci Ser C 270:1901–1904
Kawski A (1964) Dipole moments of some naphthols in the basis and stimulus condition. Nat Sci 51:82–83
Biradar DS, Siddlingeshwar B, Hanagodimath SM (2008) Estimation of ground and excited state dipole moments of some laser dyes. J Mol Struct 875:108–112
Nowak K, Wysocki S (2008) An experimental and theoretical study of dipole moments of N-[4-(9-acridinylamino)-3-methoxyphenyl]methanesulfonamide. Spectrochim Acta A 70:805–810
Sharma N, Jain SK, Rastogi RC (2007) Solvatochromic study of excited state dipole moments of some biologically active indoles and tryptamines. Spectrochim Acta A 66:171–176
Reichardt C (2003) Solvent and solvent effects in organic chemistry third and enlarged edition. Wiley VCH Verlag GmbH & Co. KGaA, Weinheim
Acknowledgments
The authors (Prashant Umape and Yogesh Gawale) are thankful to UGC for providing fellowship under UGC-SAP programme.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1522 kb)
Rights and permissions
About this article
Cite this article
Umape, P.G., Gawale, Y. & Sekar, N. Fluorescent Styryl Dyes Based on Novel 4-Methoxy-9-Methyi-9H-Carbazole-3-Carbaldehyde—Synthesis, Photophysical Properties and DFT Computations. J Fluoresc 24, 1087–1098 (2014). https://doi.org/10.1007/s10895-014-1389-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-014-1389-9