Abstract
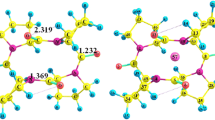
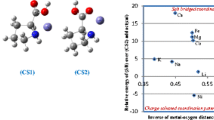
A density functional theory based on interaction of alkali metal cations (Li+, Na+, K+, Rb+ and Cs+) with cyclic peptides constructed from 3 or 4 alanine molecule (CyAla3 and CyAla4), has been investigated using mixed basis set (C, H, O, Li+, Na+ and K+ using 6-31+G(d), and the heavier cations: Rb+ and Cs+ using LANL2DZ). The minimum energy structures, binding energies, and various thermodynamic parameters of free ligands and their metal cations complexes have been determined with B3LYP and CAM-B3LYP functionals. The order of interaction energies were found to be Li+ > K+ > Na+ > Rb+ > Cs+ and Li+ > Na+ > K+ ≫ Rb+ > Cs+, calculated at CAM-B3LYP level for the M/CyAla3 and M/CyAla4 complexes, respectively. Their selectivity trend shows that the highest cation selectivity for Li+ over other alkali metal ions has been achieved on the basis of thermodynamic analysis. The main types of driving force host–guest interactions are investigated, the electron-donating O offers lone pair electrons to the contacting LP* of alkali metal cations.






Similar content being viewed by others
References
Chen, G., Su, S., Liu, R.: Theoretical studies of monomer and dimer of cyclo[(−l-Phe1-d-Ala2−)n] and cyclo[(−l-Phe1-d-MeN-Ala2−)n] (n = 3–6). J. Phys. Chem. B 106(7), 1570–1575 (2002)
Ghadiri, M.R., Granja, J.R., Buehler, L.K.: Artificial transmembrane ion channels from self-assembling peptide nanotubes. Nature 369(6478), 301–304 (1994)
Ghadiri, M.R., Granja, J.R., Milligan, R.A., McRee, D.E., Khazanovich, N.: Self-assembling organic nanotubes based on a cyclic peptide architecture. Nature 366(6453), 324–327 (1993)
Ghadiri, M.R., Kobayashi, K., Granja, J.R., Chadha, R.K., McRee, D.E.: The structural and thermodynamic basis for the formation of self-assembled peptide nanotubes. Angew. Chem. Int. Ed. Engl. 34(1), 93–95 (1995)
Hartgerink, J.D., Granja, J.R., Milligan, R.A., Ghadiri, M.R.: Self-assembling peptide nanotubes. J. Am. Chem. Soc. 118(1), 43–50 (1996)
Kobayashi, K., Granja, J.R., Ghadiri, M.R.: β-Sheet peptide architecture: measuring the relative stability of parallel vs. antiparallel β-sheets. Angew. Chem. Int. Ed. Engl. 34(1), 95–98 (1995)
Poteau, R., Trinquier, G.: All-cis cyclic peptides. J. Am. Chem. Soc. 127(40), 13875–13889 (2005)
Tan, H., Qu, W., Chen, G., Liu, R.: Theoretical investigation of the self-assembly of cyclo[(−β3-HGly)4−]. Chem. Phys. Lett. 369(5–6), 556–562 (2003)
Teranishi, M., Okamoto, H., Takeda, K., Nomura, K.-i., Nakano, A., Kalia, R.K., Vashishta, P., Shimojo, F.: Molecular dynamical approach to the conformational transition in peptide nanorings and nanotubes. J. Phys. Chem. B 113(5), 1473–1484 (2009)
Bagheri, M., Keller, S., Dathe, M.: Interaction of W-substituted analogs of cyclo-RRRWFW with bacterial lipopolysaccharides: the role of the aromatic cluster in antimicrobial activity. Antimicrob. Agents Chemother. 55(2), 788–797 (2011)
Vollenbroich, D., Özel, M., Vater, J., Kamp, R.M., Pauli, G.: Mechanism of inactivation of enveloped viruses by the biosurfactant surfactin from Bacillus subtilis. Biologicals 25(3), 289–297 (1997)
Kracht, M., Rokos, H., Ozel, M., Kowall, M., Pauli, G., Vater, J.: Antiviral and hemolytic activities of surfactin isoforms and their methyl ester derivatives. J. Antibiot. 52(7), 613–619 (1999)
Tendulkar, S.R., Saikumari, Y.K., Patel, V., Raghotama, S., Munshi, T.K., Balaram, P., Chattoo, B.B.: Isolation, purification and characterization of an antifungal molecule produced by Bacillus licheniformis BC98, and its effect on phytopathogen Magnaporthe grisea. J. Appl. Microbiol. 103(6), 2331–2339 (2007)
Weber, C., Wider, G., von Freyberg, B., Traber, R., Braun, W., Widmer, H., Wuthrich, K.: The NMR structure of cyclosporin A bound to cyclophilin in aqueous solution. Biochemistry 30(26), 6563–6574 (1991)
Trevisan, G., Maldaner, G., Velloso, N.A., Sant’Anna Gda, S., Ilha, V., Velho Gewehr Cde, C., Rubin, M.A., Morel, A.F., Ferreira, J.: Antinociceptive effects of 14-membered cyclopeptide alkaloids. J. Nat. Prod. 72(4), 608–612 (2009)
Gang, H.Z., Liu, J.F., Mu, B.Z.: Molecular dynamics simulation of surfactin derivatives at the decane/water interface at low surface coverage. J. Phys. Chem. B 114(8), 2728–2737 (2010)
Banerjee, A., Yadav, A.: Self-assembling cyclic systems as drug carriers. Appl. Nanosci. 1–14 (2012). doi:10.1007/s13204-012-0154-0
Liu, J., Fan, J., Tang, M., Zhou, W.: Molecular dynamics simulation for the structure of the water chain in a transmembrane peptide nanotube. J. Phys. Chem. A 114(6), 2376–2383 (2010)
Jishi, R.A., Flores, R.M., Valderrama, M., Lou, L., Bragin, J.: Equilibrium geometry and properties of cyclo[(Gly-d-Ala)4] and {cyclo[(Gly-d-Ala)4]}2 from density functional theory. J. Phys. Chem. A 102(48), 9858–9862 (1998)
Vijayaraj, R., Sundar Raman, S., Mahesh Kumar, R., Subramanian, V.: Studies on the structure and stability of cyclic peptide based nanotubes using oligomeric approach: a computational chemistry investigation. J. Phys. Chem. B 114(49), 16574–16583 (2010)
Vijayaraj, R., Van Damme, S., Bultinck, P., Subramanian, V.: Structure and stability of cyclic peptide based nanotubes: a molecular dynamics study of the influence of amino acid composition. Phys. Chem. Chem. Phys. 14(43), 15135–15144 (2012)
Żero, P., Pluciński, F., Mazurek, A.P.: Theoretical comparison of molecular properties of linear and cyclic glycine derived peptides and their phosphor analogues. J. Mol. Struct. (Theochem.) 915(1–3), 182–187 (2009)
Ali, S.M., Maity, D.K., De, S., Shenoi, M.R.K.: Ligands for selective metal ion extraction: a molecular modeling approach. Desalination 232(1–3), 181–190 (2008)
Casanovas, J., Rodríguez-Ropero, F., Zanuy, D., Alemán, C.: Microscopic details of the sensing ability of 15-crown-5-ether functionalized poly(bithiophene). Polymer 51(18), 4267–4272 (2010)
Hill, S.E., Feller, D.: Theoretical study of cation/ether complexes: 15-crown-5 and its alkali metal complexes. Int. J. Mass Spectrom. 201(1–3), 41–58 (2000)
Hong, J., Cho, S., Ham, S.: Metal ion shuttling mechanism through thiacalix[4]crown: a computational study. Tetrahedron Lett. 53(15), 2009–2012 (2012)
Hou, H., Zeng, X., Liu, X.: DFT study of a series of crown-4 ethers and their selectivity trend for alkali metal cations: Li+ and Na+. J. Mol. Model. 15(2), 105–111 (2009)
Becke, A.D.: Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98(7), 5648–5652 (1993)
Kamiya, M., Tsuneda, T., Hirao, K.: A density functional study of van der Waals interactions. J. Chem. Phys. 117(13), 6010–6015 (2002)
Lee, C., Yang, W., Parr, R.G.: Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37(2), 785–789 (1988)
Stephens, P.J., Devlin, F.J., Chabalowski, C.F., Frisch, M.J.: Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 98(45), 11623–11627 (1994)
Yanai, T., Tew, D.P., Handy, N.C.: A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 393(1–3), 51–57 (2004)
Frisch, M.J.T., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery Jr., J.A., Peralta, J.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, J.M., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, Ö., Foresman, J.B., Ortiz, J.V., Cioslowski, J., Fox, D.J.: Gaussian 09, Revision A.02. Gaussian Inc, Wallingford (2009)
Foster, J.P., Weinhold, F.: Natural hybrid orbitals. J. Am. Chem. Soc. 102(24), 7211–7218 (1980)
Reed, A.E., Curtiss, L.A., Weinhold, F.: Intermolecular interactions from a natural bond orbital, donor–acceptor viewpoint. Chem. Rev. 88(6), 899–926 (1988)
Kubik, S.: Large increase in cation binding affinity of artificial cyclopeptide receptors by an allosteric effect. J. Am. Chem. Soc. 121(25), 5846–5855 (1999)
De, S., Boda, A., Ali, S.M.: Preferential interaction of charged alkali metal ions (guest) within a narrow cavity of cyclic crown ethers (neutral host): a quantum chemical investigation. J. Mol. Struct. (Theochem.) 941(1–3), 90–101 (2010)
Kubik, S., Goddard, R.: Intramolecular conformational control in a cyclic peptide composed of alternating -proline and substituted 3-aminobenzoic acid subunits. Chem. Commun. 0(7), 633–634 (2000)
Praveena, G., Kolandaivel, P.: Interaction of metal ions with cyclo[(1R,3S)-γ-Acc-Gly]3 hexapeptide—a theoretical study. J. Mol. Struct. (Theochem.) 900(1–3), 96–102 (2009)
Acknowledgments
We would like to acknowledge the Isfahan University of Technology for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Najafi Chermahini, A., Rezapour, M. & Teimouri, A. Selective complexation of alkali metal ions and nanotubular cyclopeptides: a DFT study. J Incl Phenom Macrocycl Chem 79, 205–214 (2014). https://doi.org/10.1007/s10847-013-0346-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-013-0346-6