Abstract
Purpose
The purpose of the present study was to investigate the epigenetic mechanisms responsible for the aberrant aromatase expression (CYP19A1) in Cumulus Cells (CCs) of infertile endometriosis patients.
Method
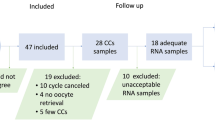
Cumulus cells were obtained from 24 infertile patients with and without endometriosis who underwent ovarian stimulation for intracytoplasmic sperm injection. Expression of CYP19A1 gene was quantified using Reverse Transcription Q-PCR. DNA methylation, histone modifications, and binding of Estrogen Receptor, ERβ to regulatory DNA sequences of CYP19A1 gene were evaluated by Chromatin ImmunoPrecipitation (ChIP) assay.
Results
CYP19A1 gene expression in CCs of endometriosis patients was significantly lower than the control group (P = 0.04). Higher incorporation of MeCP2 (as a marker of DNA methylation) on PII and PI.4 promoters, and hypoacetylation at H3K9 in PII and hypermethylation at H3K9 in PI.4 were observed in CYP19A1 gene in endometriosis patients (P < 0.05). Moreover, a decreased level of ERβ binding to PII and an increased level of its binding to PI.3 and PI.4 promoters of CYP19A1 were observed in endometriosis patients when compared to control.
Conclusion
Significant reduction of CYP19A1 gene expression in CCs of endometriosis patients may be the result of epigenetic alterations in its regulatory regions, either by DNA methylation or histone modifications. These epigenetic changes along with differential binding of ERβ (as a transcription factor) in CYP19A1 promoters may impair follicular steroidogenesis, leading to poor Oocyte and embryo condition in endometriosis patients.


Similar content being viewed by others
References
Kennedy S, Bergqvist A, Chapron C, D’Hooghe T, Dunselman G, Greb R, et al. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20(10):2698–704.
Dunselman G, Vermeulen N, Becker C, Calhaz-Jorge C, D’Hooghe T, De Bie B, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–12.
Gupta S, Goldberg JM, Aziz N, Goldberg E, Krajcir N, Agarwal A. Pathogenic mechanisms in endometriosis-associated infertility. Fertil Steril. 2008;90(2):247–57.
Garrido Ns, Navarro J, Remohà J, SimÃ3n C, Pellicer A. Follicular hormonal environment and embryo quality in women with endometriosis. Human reproduction update 2000;6(1):67-74.
Hsu AL, Townsend PM, Oehninger S, Castora FJ. Endometriosis may be associated with mitochondrial dysfunction in cumulus cells from subjects undergoing in vitro fertilization-intracytoplasmic sperm injection, as reflected by decreased adenosine triphosphate production. Fertil Stril. 2015;103(2):347–52. e1.
Wang J, Shen X, Huang X, Zhao Z. Follicular fluid levels of prostaglandin E2 and the effect of prostaglandin E2 on steroidogenesis in granulosa-lutein cells in women with moderate and severe endometriosis undergoing in vitro fertilization and embryo transfer. Chin Med J. 2012;125(22):3985–90.
Lessey BA, Young SL. Pathophysiology of infertility in endometriosis. Endometriosis: Science and Practice 2012:240-54. doi:10.1002/9781444398519.Ch23.
Pellicer A, Oliveira N, Ruiz A, Remohí J, Simón C. Exploring the mechanism(s) of endometriosis-related infertility: an analysis of embryo development and implantation in assisted reproduction. Hum Reprod. 1995;10 suppl 2:91–7.
Albertini DF, Combelles C, Benecchi E, Carabatsos MJ. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction. 2001;121(5):647–53.
Huang Z, Wells D. The human oocyte and cumulus cells relationship: new insights from the cumulus cell transcriptome. Mol Hum Reprod. 2010;16(10):715–25.
Egea RR, Puchalt NG, Escrivá MM, Varghese AC. OMICS: current and future perspectives in reproductive medicine and technology. J Hum Reprod Sci. 2014;7(2):73.
Gasca S, Pellestor F, Assou S, Loup V, Anahory T, Dechaud H, et al. Identifying new human oocyte marker genes: a microarray approach. Reprod Biomed Online. 2007;14(2):175–83.
Hamel M, Dufort I, Robert C, Gravel C, Leveille M-C, Leader A, et al. Identification of differentially expressed markers in human follicular cells associated with competent oocytes. Hum Reprod. 2008;23(5):1118–27.
Feuerstein P, Cadoret V, Dalbies-Tran R, Guerif F, Bidault R, Royere D. Gene expression in human cumulus cells: one approach to oocyte competence. Hum Reprod. 2007;22(12):3069–77.
Lucidi P, BernabÃ2 N, Turriani M, Barboni B, Mattioli M. Cumulus cells steroidogenesis is influenced by the degree of oocyte maturation. Reprod Biol Endocrinol 2003;1:45.
Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci. 1998;95(12):6965–70.
Pelletier G, El-Alfy M. Immunocytochemical localization of estrogen receptors α and β in the human reproductive organs. J Clin Endocrinol Metab. 2000;85(12):4835–40.
Marino M, Galluzzo P, Ascenzi P. Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics. 2006;7(8):497.
Zhuang L-Z, Adashi EY, Hsueh AJ. Direct enhancement of gonadotropin-stimulated ovarian estrogen biosynthesis by estrogen and clomiphene citrate. Endocrinology. 1982;110(6):2219–21.
Katz-Jaffe MG, Surrey ES, Minjarez DA, Gustofson RL, Stevens JM, Schoolcraft WB. Association of abnormal ovarian reserve parameters with a higher incidence of aneuploid blastocysts. Obstet Gynecol. 2013;121(1):71–7.
Conley A, Mapes S, Corbin C, Greger D, Walters K, Trant J, et al. A comparative approach to structure-function studies of mammalian aromatases. J Steroid Biochem Mol Biol. 2001;79(1):289–97.
Sebastian S, Bulun SE. A highly complex organization of the regulatory region of the human CYP19 (aromatase) gene revealed by the human genome project. J Clin Endocrinol Metab. 2001;86(10):4600–2.
Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57(3):359–83.
Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994;15(3):342–55.
Giudice LC, Kao LC. Endometriosis. Lancet. 2015;364(9447):1789–99.
Barcelos IDE, Donabella FC, Ribas CP, Meola J, Ferriani RA, de Paz CCP, et al. Down-regulation of the CYP19A1 gene in cumulus cells of infertile women with endometriosis. RBM Online. 2015;30(5):532–41.
Magli MC, Jones GM, Lundin K, Van den Abbeel E. Atlas of human embryology: from oocytes to preimplantation embryos. Hum Reprod. 2012;27:1.
Mönkkönen KS, Aflatoonian R, Lee K-F, Yeung WS, Tsao S-W, Laitinen JT, et al. Localization and variable expression of Gαi2 in human endometrium and fallopian tubes. Hum Reprod. 2007;22(5):1224–30.
Mönkkönen KS, Aflatoonian R, Lee K-F, Yeung WS, Tsao S-W, Laitinen JT, et al. Hormonal regulation of Gαi2 and mPRα in immortalized human oviductal cell line OE-E6/E7. MHR. 2007;13(12):845–51.
Stocco C. Aromatase expression in the ovary: hormonal and molecular regulation. Steroids. 2008;73(5):473–87.
Lu X, Wu Y, Gao X-H, Wang Y-W, Wang L, Sun X-X. Effect of letrozole on estradiol production and P450 aromatase messenger RNA expression of cultured luteinized granulosa cells from women with and without endometriosis. Fertil Steril. 2012;98(1):131–5.
Harlow C, Cahill D, Maile L, Talbot W, Mears J, Wardle P, et al. Reduced preovulatory granulosa cell steroidogenesis in women with endometriosis. J Clin Endocrinol Metab. 1996;81(1):426–9.
De Abreu LG, Romã£O GS, Reis RMD, Ferriani RA, de Sã¡ MFS, Moura MD. Reduced aromatase activity in granulosa cells of women with endometriosis undergoing assisted reproduction techniques. Gynecol Endocrinol. 2006;22(8):432–6.
De Abreu LG, Silveira VS, Scrideli CA, Ramos ES, Dos Reis RM, Ferriani RA, et al. Endometriosis does not alter aromatase gene expression (CYP19A1) in mural lutein-granulosa cells of women undergoing assisted reproduction techniques—a pilot study. J Endometriosis. 2011;3(4):177–82.
Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31(1):27–36.
Nan X, Ng H-H, Johnson CA, Laherty CD, Turner BM, Eisenman RN, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393(6683):386–9.
Lewis JD, Meehan RR, Henzel WJ, Maurer-Fogy I, Jeppesen P, Klein F, et al. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell. 1992;69(6):905–14.
Fo F, Hurd PJ, Wolf D, Nan X, Bird AP, Kouzarides T. The methyl-CpG-binding protein MeCP2 links DNA methylation to histone methylation. J Biol Chem. 2003;278(6):4035–40.
Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128(4):707–19.
Fischer JJ, Toedling J, Krueger T, Schueler M, Huber W, Sperling S. Combinatorial effects of four histone modifications in transcription and differentiation. Genomics. 2008;91(1):41–51.
Adashi E, Hsueh A. Estrogens augment the stimulation of ovarian aromatase activity by follicle-stimulating hormone in cultured rat granulosa cells. J Biol Chem. 1982;257(11):6077–83.
Klinge CM. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001;29(14):2905–19.
Bréard E, Roussel H, Lindet Y, Mittre H, Leymarie P. Presence of exon I. 4 mRNA from CYP19 gene in human granulosa cells. Mol Cell Endocrinol. 1999;154(1):187–90.
Hervouet E, Cartron P-F, Jouvenot M, Delage-Mourroux R. Epigenetic regulation of estrogen signaling in breast cancer. Epigenetics. 2013;8(3):237–45.
Dago DN, Scafoglio C, Rinaldi A, Memoli D, Giurato G, Nassa G, et al. Estrogen receptor beta impacts hormone-induced alternative mRNA splicing in breast cancer cells. BMC Genomics. 2015;16(1):367.
Bulun SE, Monsivais D, Kakinuma T, Furukawa Y, Bernardi L, Pavone ME, et al. Molecular biology of endometriosis: from aromatase to genomic abnormalities. Semin Reprod Med. 2015;2015:220–4.
Mahdian S, Aflatoonian R, Yazdi RS, Yaghmaei P, Ramazanali F, Afsharian P, et al. Macrophage migration inhibitory factor as a potential biomarker of endometriosis. Fertil Steril. 2015;103(1):153–9. e3.
Simon C, Gutierrez A, Vidal A, De los Santos M, Tarin J, Remohi J, et al. Outcome of patients with endometriosis in assisted reproduction: results from in-vitro fertilization and oocyte donation. Hum Reprod. 1994;9(4):725–9.
Pellicer A, Navarro J, Bosch E, Garrido N, Garcia‐Velasco JA, Remohí J, et al. Endometrial quality in infertile women with endometriosis. Ann N Y Acad Sci. 2001;943(1):122–30.
Hammes SR. Steroids and oocyte maturation—a new look at an old story. Mol Endocrinol. 2004;18(4):769–75.
Tesarik J, Mendoza C. Nongenomic effects of 17 beta-estradiol on maturing human oocytes: relationship to oocyte developmental potential. J Clin Endocrinol Metab. 1995;80(4):1438–43.
Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL, Schoolcraft WB. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil Stril. 2015;103(2):303–16.
Neal MS, Younglai EV, Holloway AC, Foster WG. Aromatase activity in granulosa cells as a predictor of pregnancy potential. International Congress Series; 2004: Elsevier B.V.; 2004. p. 139–42. doi:10.1016/j.ics 2004.05.022.
Peña JE, Chang PL, Chan L-K, Zeitoun K, Thornton MH, Sauer MV. Supraphysiological estradiol levels do not affect oocyte and embryo quality in oocyte donation cycles. Hum Reprod. 2002;17(1):83–7.
Brizek CL, Schlaff S, Pellegrini VA, Frank JB, Worrilow KC. Increased incidence of aberrant morphological phenotypes in human embryogenesis—an association with endometriosis. J Assist Reprod Genet. 1995;12(2):106–12.
Sanchez AM, Somigliana E, Vercellini P, Pagliardini L, Candiani M, Vigano P. Endometriosis as a detrimental condition for granulosa cell steroidogenesis and development: from molecular alterations to clinical impact. J Steroid Biochem Mol Biol. 2016;155:35–46.
Acknowledgments
The authors thank all the patients that consented to participate in this study and the embryologists at Royan institute, Tehran, Iran., especially Dr. Bahar Movaghar and Dr. Poopak Eftekhari Yazdi, for their help with patient recruitment and associated embryology. Further, the authors would like to acknowledge Mrs. Raha Favaedi, Miss Samaneh Aghajanpour, and Mrs. Neda Soltani for their skilful technical assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This cross-sectional study was approved by the Ethics Committees of Iran University of Medical Sciences (IUMS, no: 23108-April 2014) and the Royan Institute. Also, written informed consent was obtained from all case and control subjects prior to the oocyte retrieval.
Conflict of interest
There is no conflict of interest in this study.
Funding
This research was supported by the Vice Chancellor of Research at Iran University of Medical Sciences and Royan institute, Tehran, Iran.
Additional information
Capsule
These epigenetic changes along with differential binding of ERβ (as a transcription factor) in CYP19A1 promoters may impair follicular steroidogenesis, leading to poor Oocyte and embryo condition in endometriosis patients.
Rights and permissions
About this article
Cite this article
Hosseini, E., Mehraein, F., Shahhoseini, M. et al. Epigenetic alterations of CYP19A1 gene in Cumulus cells and its relevance to infertility in endometriosis. J Assist Reprod Genet 33, 1105–1113 (2016). https://doi.org/10.1007/s10815-016-0727-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0727-z