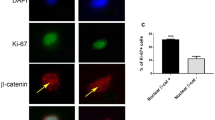
Abstract
Hoxa2 gene was reported to be expressed by oligodendrocytes (OLs) and down-regulated at the terminal differentiation stage during oligodendrogenesis in mice (Nicolay et al. 2004b). To further investigate the role of Hoxa2 in oligodendroglial development, a tetracycline regulated controllable expression system was utilized to establish a stable cell line (CG4-SHoxa2 [sense Hoxa2]), where the expression level of Hoxa2 gene could be up-regulated. The impact of Hoxa2 over-expression on the proliferation and differentiation of CG4-SHoxa2 cells was investigated. Up-regulation of Hoxa2 increased the proliferation of CG4-SHoxa2 cells. The mRNA levels of PDGFαR (platelet-derived growth factor [PDGF] alpha receptor), which is expressed by OL progenitor cells, were not different in CG4-SHoxa2 cells compared to wild-type CG4 cells. Semi-quantitative RT-PCR revealed that the mRNA levels of myelin basic protein (MBP) was lower in CG4-SHoxa2 cells than in wild-type CG4 cells indicating the differentiation of CG4-SHoxa2 cells was delayed when the Hoxa2 gene was up-regulated.




Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- A–P:
-
Anterior–posterior
- BrdU:
-
Bromodeoxyuridine
- CDK:
-
Cyclin-dependent protein kinase
- CNS:
-
Central nervous system
- DM:
-
Differentiation medium
- DMEM:
-
Dulbecco’s modified eagle medium
- Dox:
-
Doxycycline
- EDTA:
-
Ethylenediaminetetraacetic acid
- ES:
-
Embryonic stem cells
- FITC:
-
Fluorescein isothiocyanate
- GalC:
-
Galactocerebroside C
- GM:
-
Growth medium
- Hyg:
-
Hygromycin
- MBP:
-
Myelin basic protein
- mRNA:
-
Messenger ribonucleic acid
- Myc:
-
c-myc gene/protein
- Nkx2.2:
-
NK2 transcription factor related, locus 2
- Nkx6.1:
-
NK6 transcription factor related, locus 1
- O-2A:
-
Oligodendrocyte-type 2 astrocyte
- OL(s):
-
Oligodendrocyte(s)
- Olig1/2:
-
Oligodendrocyte lineage gene 1/2
- OPC(s):
-
Oligodendrocyte precursor cell(s)
- p27/Kip1:
-
Kinase inhibitory protein 1
- PBS:
-
Phosphate buffered saline
- PDGF:
-
Platelet-derived growth factor
- PDGFαR:
-
Platelet-derived growth factor receptor α
- RIPA:
-
Radioimmunoprecipitation assay
- RT:
-
Room temperature
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- SHoxa2:
-
Sense Hoxa2
- Sox:
-
SRY-box containing gene
- Taq :
-
Thermus aquaticus
- Tet:
-
Tetracycline
- tTA:
-
Tetracycline transcriptional activator
- TF(s):
-
Transcription factor(s)
References
Akin ZN, Nazarali AJ (2005) Hox genes and their candidate downstream targets in the developing central nervous system. Cell Mol Neurobiol 25:697–741
Arenkiel BR, Gaufo GO, Capecchi MR (2003) Hoxb1 neural crest preferentially form glia of the PNS. Dev Dyn 227:379–386
Arenkiel BR, Tvrdik P, Gaufo GO, Capecchi MR (2004) Hoxb1 functions in both motoneurons and in tissues of the periphery to establish and maintain the proper neuronal circuitry. Genes Dev 18:1539–1552
Barber BA, Rastegar M (2010) Epigenetic control of Hox genes during neurogenesis, development, and disease. Ann Anat 192:261–274
Baron W, Shattil SJ, ffrench-Constant C (2002) The oligodendrocyte precursor mitogen PDGF stimulates proliferation by activation of alpha(v)beta3 integrins. EMBO J 21:1957–1966
Bhat NR, Zhang P (1999) Hydrogen peroxide activation of multiple mitogen-activated protein kinases in an oligodendrocyte cell line: role of extracellular signal-regulated kinase in hydrogen peroxide-induced cell death. J Neurochem 72:112–119
Booth J, Nicolay DJ, Doucette JR, Nazarali AJ (2007) Hoxd1 is expressed by oligodendroglial cells and binds to a region of the human myelin oligodendrocyte glycoprotein promoter in vitro. Cell Mol Neurobiol 27:641–650
Briscoe J, Wilkinson DG (2004) Establishing neuronal circuitry: Hox genes make the connection. Genes Dev 18:1643–1648
Bromleigh VC, Freedman LP (2000) p21 is a transcriptional target of HOXA10 in differentiating myelomonocytic cells. Genes Dev 14:2581–2586
Cai J, Qi Y, Hu X, Tan M, Liu Z, Zhang J, Li Q, Sander M, Qiu M (2005) Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of Nkx6 regulation and Shh signaling. Neuron 45:41–53
Care A, Testa U, Bassani A, Tritarelli E, Montesoro E, Samoggia P, Cianetti L, Peschle C (1994) Coordinate expression and proliferative role of HOXB genes in activated adult T lymphocytes. Mol Cell Biol 14:4872–4877
Compston A (2004) The pathogenesis and basis for treatment in multiple sclerosis. Clin Neurol Neurosurg 106:246–248
Crickmore MA, Mann RS (2006) Hox control of organ size by regulation of morphogen production and mobility. Science 313:63–68
Dasen JS, Tice BC, Brenner-Morton S, Jessell TM (2005) A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell 123:477–491
Del Bene F, Wittbrodt J (2005) Cell cycle control by homeobox genes in development and disease. Semin Cell Dev Biol 16:449–460
Doucette R, Devon R (1994) Media that support the growth and differentiation of oligodendrocytes do not induce olfactory ensheathing cells to express a myelinating phenotype. Glia 10:296–310
Doucette R, Jiao R, Nazarali A (2010) Age-related and cuprizone-induced changes in myelin and transcription factor gene expression and in oligodendrocyte cell densities in the rostral corpus callosum of mice. Cell Mol Neurobiol 30:607–629
Du Y, Dreyfus CF (2002) Oligodendrocytes as providers of growth factors. J Neurosci Res 68:647–654
Dubrulle J, Pourquie O (2002) From head to tail: links between the segmentation clock and antero-posterior patterning of the embryo. Curr Opin Genet Dev 12:519–523
Ellison JA, de Vellis J (1994) Platelet-derived growth factor receptor is expressed by cells in the early oligodendrocyte lineage. J Neurosci Res 37:116–128
Emery B (2010) Regulation of oligodendrocyte differentiation and myelination. Science 330:779–782
Engel U, Wolswijk G (1996) Oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells derived from adult rat spinal cord: in vitro characteristics and response to PDGF, bFGF and NT-3. Glia 16:16–26
Espinosa de los Monteros A, Zhao P, Huang C, Pan T, Chang R, Nazarian R, Espejo D, de Vellis J (1997) Transplantation of CG4 oligodendrocyte progenitor cells in the myelin-deficient rat brain results in myelination of axons and enhanced oligodendroglial markers. J Neurosci Res 50:872–887
Fields RD (2005) Myelination: an overlooked mechanism of synaptic plasticity? Neuroscientist 11:528–531
Fisher D, Méchali M (2003) Vertebrate HoxB gene expression requires DNA replication. EMBO J 22:3737–3748
Ford HL (1998) Homeobox genes: a link between development, cell cycle, and cancer? Cell Biol Int 22:397–400
Franklin RJ, Bayley SA, Milner R, Ffrench-Constant C, Blakemore WF (1995) Differentiation of the O-2A progenitor cell line CG-4 into oligodendrocytes and astrocytes following transplantation into glia-deficient areas of CNS white matter. Glia 13:39–44
Franklin RJ, Bayley SA, Blakemore WF (1996) Transplanted CG4 cells (an oligodendrocyte progenitor cell line) survive, migrate, and contribute to repair of areas of demyelination in X-irradiated and damaged spinal cord but not in normal spinal cord. Exp Neurol 137:263–276
Fu H, Qi Y, Tan M, Cai J, Takebayashi H, Nakafuku M, Richardson W, Qiu M (2002) Dual origin of spinal oligodendrocyte progenitors and evidence for the cooperative role of Olig2 and Nkx2.2 in the control of oligodendrocyte differentiation. Development 129:681–693
Gabellini D, Colaluca IN, Vodermaier HC, Biamonti G, Giacca M, Falaschi A, Riva S, Peverali FA (2003) Early mitotic degradation of the homeoprotein HOXC10 is potentially linked to cell cycle progression. EMBO J 22:3715–3724
Gard AL, Pfeiffer SE (1990) Two proliferative stages of the oligodendrocyte lineage (A2B5+O4- and O4+GalC-) under different mitogenic control. Neuron 5:615–625
Gaufo GO, Wu S, Capecchi MR (2004) Contribution of Hox genes to the diversity of the hindbrain sensory system. Development 131:1259–1266
Gendron-Maguire M, Mallo M, Zhang M, Gridley T (1993) Hoxa-2 mutant mice exhibit homeotic transformation of skeletal elements derived from cranial neural crest. Cell 75:1317–1331
Gokhan S, Marin-Husstege M, Yung SY, Fontanez D, Casaccia-Bonnefil P, Mehler MF (2005) Combinatorial profiles of oligodendrocyte-selective classes of transcriptional regulators differentially modulate myelin basic protein gene expression. J Neurosci 25:8311–8321
Hao Z, Yeung J, Wolf L, Doucette R, Nazarali A (1999) Differential expression of Hoxa-2 protein along the dorsal-ventral axis of the developing and adult mouse spinal cord. Dev Dyn 216:201–217
He H, Hua X, Yan J (2011) Epigenetic regulations in hematopoietic Hox code. Oncogene 30:379–388
Hu JG, Fu SL, Zhang KH, Li Y, Yin L, Lu PH, Xu XM (2004) Differential gene expression in neural stem cells and oligodendrocyte precursor cells: a cDNA microarray analysis. J Neurosci Res 78:637–646
Hueber SD, Lohmann I (2008) Shaping segments: Hox gene function in the genomic age. Bioessays 30:965–979
Lagarde WH, Benjamin R, Heerens AT, Ye P, Cohen RI, Moats-Staats BM, D’Ercole AJ (2007) A non-transformed oligodendrocyte precursor cell line, OL-1, facilitates studies of insulin-like growth factor-I signaling during oligodendrocyte development. Int J Dev Neurosci 25:95–105
Levine EM, Green ES (2004) Cell-intrinsic regulators of proliferation in vertebrate retinal progenitors. Semin Cell Dev Biol 15:63–74
Louis JC, Magal E, Muir D, Manthorpe M, Varon S (1992) CG-4, a new bipotential glial cell line from rat brain, is capable of differentiating in vitro into either mature oligodendrocytes or type-2 astrocytes. J Neurosci Res 31:193–204
Lu QR, Yuk D, Alberta JA, Zhu Z, Pawlitzky I, Chan J, McMahon AP, Stiles CD, Rowitch DH (2000) Sonic hedgehog-regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron 25:317–329
Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH (2002) Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell 109:75–86
Ludwin SK (1997) The pathobiology of the oligodendrocyte. J Neuropathol Exp Neurol 56:111–124
Lumsden A, Krumlauf R (1996) Patterning the vertebrate neuraxis. Science 274:1109–1115
Luo L, Yang X, Takihara Y, Knoetgen H, Kessel M (2004) The cell-cycle regulator geminin inhibits Hox function through direct and polycomb-mediated interactions. Nature 427:749–753
Maconochie M, Nonchev S, Morrison A, Krumlauf R (1996) Paralogous Hox genes: function and regulation. Annu Rev Genet 30:529–556
Magli MC, Barba P, Celetti A, De Vita G, Cillo C, Boncinelli E (1991) Coordinate regulation of HOX genes in human hematopoietic cells. Proc Natl Acad Sci USA 88:6348–6352
Mallo M, Wellik DM, Deschamps J (2010) Hox genes and regional patterning of the vertebrate body plan. Dev Biol 344:7–15
Marshall H, Nonchev S, Sham MH, Muchamore I, Lumsden A, Krumlauf R (1992) Retinoic acid alters hindbrain Hox code and induces transformation of rhombomeres 2/3 into a 4/5 identity. Nature 360:737–741
Marshall H, Morrison A, Studer M, Pöpperl H, Krumlauf R (1996) Retinoids and Hox genes. FASEB J 10:969–978
Martinez-Ceballos E, Chambon P, Gudas LJ (2005) Differences in gene expression between wild-type and Hoxa1 knockout embryonic stem cells after retinoic acid treatment or leukemia inhibitory factor (LIF) removal. J Biol Chem 280:16484–16498
Miskimins R, Srinivasan R, Marin-Husstege M, Miskimins WK, Casaccia-Bonnefil P (2002) p27Kip1 enhances myelin basic protein gene promoter activity. J Neurosci Res 67:100–105
Narita Y, Rijli FM (2009) Hox genes in neural patterning and circuit formation in the mouse hindbrain. Curr Top Dev Biol 88:139–167
Nave KA (2010) Myelination and support of axonal integrity by glia. Nature 468:244–252
Nazarali A, Kim Y, Nirenberg M (1992) Hox-1.11 and Hox-4.9 homeobox genes. Proc Natl Acad Sci USA 89:2883–2887
Nicolay DJ, Doucette JR, Nazarali AJ (2004a) Hoxb4 in oligodendrogenesis. Cell Mol Neurobiol 24:357–366
Nicolay DJ, Doucette JR, Nazarali AJ (2004b) Early stages of oligodendrocyte development in the embryonic murine spinal cord proceed normally in the absence of Hoxa2. Glia 48:14–26
Nicolay D, Doucette R, Nazarali A (2007) Transcriptional control of oligodendrogenesis. Glia 55:1287–1299
Noll E, Miller RH (1994) Regulation of oligodendrocyte differentiation: a role for retinoic acid in the spinal cord. Development 120:649–660
Qi Y, Cai J, Wu Y, Wu R, Lee J, Fu H, Rao M, Sussel L, Rubenstein J, Qiu M (2001) Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development 128:2723–2733
Quaranta MT, Petrini M, Tritarelli E, Samoggia P, Care A, Bottero L, Testa U, Peschle C (1996) HOXB cluster genes in activated natural killer lymphocytes: expression from 3′ → 5′ cluster side and proliferative function. J Immunol 157:2462–2469
Ranscht B, Clapshaw PA, Price J, Noble M, Seifert W (1982) Development of oligodendrocytes and Schwann cells studied with a monoclonal antibody against galactocerebroside. Proc Natl Acad Sci USA 79:2709–2713
Remahl S, Hildebrand C (1990) Relation between axons and oligodendroglial cells during initial myelination I. The glial unit. J Neurocytol 19:313–328
Rijli FM, Mark M, Lakkaraju S, Dierich A, Dollé P, Chambon P (1993) A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell 75:1333–1349
Rijli FM, Gavalas A, Chambon P (1998) Segmentation and specification in the branchial region of the head: the role of the Hox selector genes. Int J Dev Biol 42:393–401
Rivers L, Young K, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson W (2008) PDGFαR/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci 11:1392–1401
Rumsby M, Suggitt F, Haynes L, Hughson E, Kidd D, McNulty S (1999) Substratum of pleiotrophin (HB-GAM) stimulates rat CG-4 line oligodendrocytes to adopt a bipolar morphology and disperse: primary O-2A progenitor glial cells disperse similarly on pleiotrophin. Glia 26:361–367
Serpente P, Tümpel S, Ghyselinck NB, Niederreither K, Wiedemann LM, Dollé P, Chambon P, Krumlauf R, Gould AP (2005) Direct crossregulation between retinoic acid receptor β and Hox genes during hindbrain segmentation. Development 132:503–513
Sommer I, Schachner M (1981) Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol 83:311–327
Takebayashi H, Yoshida S, Sugimori M, Kosako H, Kominami R, Nakafuku M, Nabeshima Y (2000) Dynamic expression of basic helix-loop-helix Olig family members: implication of Olig2 in neuron and oligodendrocyte differentiation and identification of a new member, Olig3. Mech Dev 99:143–148
Tan DP, Ferrante J, Nazarali A, Shao X, Kozak CA, Guo V, Nirenberg M (1992) Murine Hox-1.11 homeobox gene structure and expression. Proc Natl Acad Sci USA 89:6280–6284
Tontsch U, Archer DR, Dubois-Dalcq M, Duncan ID (1994) Transplantation of an oligodendrocyte cell line leading to extensive myelination. Proc Natl Acad Sci USA 91:11616–11620
Tourbah A, Linnington C, Bachelin C, Avellana-Adalid V, Wekerle H, Baron-Van Evercooren A (1997) Inflammation promotes survival and migration of the CG4 oligodendrocyte progenitors transplanted in the spinal cord of both inflammatory and demyelinated EAE rats. J Neurosci Res 50:853–861
Trainor PA, Krumlauf R (2001) Hox genes, neural crest cells and branchial arch patterning. Curr Opin Cell Biol 13:698–705
Tümpel S, Wiedemann LM, Krumlauf R (2009) Hox genes and segmentation of the vertebrate hindbrain. Curr Top Dev Biol 88:103–137
Wang L, Mear JP, Kuan CY, Colbert MC (2005) Retinoic acid induces CDK inhibitors and growth arrest specific (Gas) genes in neural crest cells. Dev Growth Differ 47:119–130
Wegner M, Stolt CC (2005) From stem cells to neurons and glia: a Soxist’s view of neural development. Trends Neurosci 28:583–588
Wei Q, Miskimins WK, Miskimins R (2005) Stage-specific expression of myelin basic protein in oligodendrocytes involves Nkx2.2-mediated repression that is relieved by the Sp1 transcription factor. J Biol Chem 280:16284–16294
Wellik DM (2009) Hox genes and vertebrate axial pattern. Curr Top Dev Biol 88:257–278
Ye P, Bagnell R, D’Ercole AJ (2003) Mouse NG2+oligodendrocyte precursors express mRNA for proteolipid protein but not its DM-20 variant: a study of laser microdissection-captured NG2+cells. J Neurosci 23:4401–4405
Yue L, Daikoku T, Hou X, Li M, Wang H, Nojima H, Dey SK, Das SK (2005) Cyclin G1 and cyclin G2 are expressed in the periimplantation mouse uterus in a cell-specific and progesterone-dependent manner: evidence for aberrant regulation with Hoxa-10 deficiency. Endocrinology 146:2424–2433
Zhang SC (2001) Defining glial cells during CNS development. Nat Rev Neurosci 2:840–843
Zhou Q, Anderson DJ (2002) The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell 109:61–73
Acknowledgments
We dedicate this manuscript to Marshall Nirenberg, who is remembered for his pioneering work in deciphering the genetic code and in whose lab AJN first cloned the Hoxa2 (Hox1.11) gene. This work was supported by an operating grant from the Canadian Institutes of Health Research (CIHR) to AJN and JRD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, M., Doucette, J.R. & Nazarali, A.J. Conditional Tet-Regulated Over-Expression of Hoxa2 in CG4 Cells Increases Their Proliferation and Delays Their Differentiation into Oligodendrocyte-like Cells Expressing Myelin Basic Protein. Cell Mol Neurobiol 31, 875–886 (2011). https://doi.org/10.1007/s10571-011-9685-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-011-9685-2