Abstract
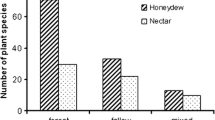
The legume Chamaecrista fasciculata attracts ants to its extrafloral nectar (EFN) which can lead to reduced herbivory and increased fecundity for the plant. In Florida, Opuntia stricta and O. humifusa, hosts of the invasive moth Cactoblastis cactorum, are often found growing in close association with C. fasciculata. We tested the hypotheses that O. stricta and O. humifusa individuals have higher ant abundance, lower levels of herbivore damage, and increased growth when growing in close association with C. fasciculata compared with individuals not growing near the plant. We also experimentally placed C. cactorum eggsticks and pupae on Opuntia individuals to see if ant predation of these stages occurred, and if so, whether predation rates were higher on individuals growing close to C. fasciculata. Opuntia plants near C. fasciculata were less likely to be attacked by C. cactorum and had higher ant abundance than plants far from C. fasciculata. Field surveys showed that Opuntia plants near C. fasciculata had a lower proportion of cladodes with C. cactorum damage of any type. Proportions of cladodes with damage from five native herbivores were not significantly different between treatments. In addition, Opuntia individuals growing near C. fasciculata added proportionately more pads during the growing season. We found evidence of ant predation on 15.9% of C. cactorum eggsticks and 17.6% of pupae. In August and October of 2008, there was significantly more evidence of predation on eggs and pupae placed on Opuntia individuals near C. fasciculata. No effect of distance to C. fasciculata was seen in November of 2008, potentially because plants were no longer producing EFN at this time. Our finding that Opuntia plants close to C. fasciculata show reduced herbivory from invasive C. cactorum, but not from the native herbivores examined, suggests that patterns of associational resistance may be influenced by the co-evolutionary history of the organisms in question.




Similar content being viewed by others
References
Abdala-Roberts L, Marquis RJ (2007) Test of local adaptation to biotic interactions and soil abiotic conditions in the ant-tended Chamaecrista fasciculata (Fabaceae). Oecologia 154:315–326
Agrawal AA, Lau JA, Hamback PA (2006) Community heterogeneity and the evolution of interactions between plants and insect herbivores. Q Rev Biol 81:349–376
Anderson EF (2001) The cactus family. Timber Press, Portland, OR
Andow DA (1991) Vegetational diversity and arthropod population response. Annu Rev Entomol 36:561–586
Atsatt PR, Odowd DJ (1976) Plant defense guilds. Science 193:24–29
Baker AJ, Stiling P (2009) Comparing the effects of the exotic cactus-feeding moth, Cactoblastis cactorum (Berg) (Lepidoptera: Pyralidae) and the native cactus-feeding moth, Melitara prodenialis (Walker) (Lepidoptera: Pyralidae) on two species of Florida Opuntia. Biol Invasions 11:619–624
Barbosa P, Caldas A (2007) Do larvae of species in macrolepidopteran assemblages share traits that influence susceptibility to parasitism? Env Entomol 36:329–336
Barbosa P, Hines J, Kaplan J, Martinson H, Szczepaniec A, Szendrei Z (2009) Associational resistance and associational susceptibility: having right or wrong neighbors. Annu Rev Ecol Evol S 40:1–20
Barton AM (1986) Spatial variation in the effect of ants on an extrafloral nectary plant. Ecology 67:495–504
Bennett FD, Habeck DH (1996) Cactoblastis cactorum: a successful weed control agent in the Caribbean, now a pest in Florida? In: Delfosse ES, Scott RR (eds) Biological control of weeds: proceedings of the VIII international symposium on biological control of weeds. CSIRO Publishing, Melbourne, pp 21–26
Bentley BL (1977) Extrafloral nectaries and protection by pugnacious bodyguards. J Ecol 65:27–38
Boecklen WJ (1984) The role of extrafloral nectaries in the herbivore defense of Cassia fasciculata. Ecol Entomol 9:243–249
Brown BJ, Ewel JJ (1987) Herbivory in complex and simple tropical successional ecosystems. Ecology 68:108–116
Carpenter JE, Bloem S, Bloem KA (2001) Inherited sterility in Cactoblastis cactorum (Lepidoptera: Pyralidae). Fla Entomol 84:537–542
Del Carmen Mandujano M, Montana C, Mendez I, Golubov J (1998) The relative contribution of sexual reproduction and clonal propagation in Opuntia rastrera from two habitats in the Chihuahuah desert. J Ecol 86:911–921
DelClaro K, Berto V, Reu W (1996) Effect of herbivore deterrence by ants on the fruit set of an extrafloral nectary plant, Qualea multiflora (Vochysiaceae). J Trop Ecol 12:887–892
Deyrup M, Davis L, Cover S (2000) Exotic ants in Florida. T Am Entomol Soc 126:293–326
Dodd AP (1940) The biological campaign against prickly pear. Commonwealth Prickly Pear Board Bulletin, Brisbane
Finch S, Billiald H, Collier RH (2003) Companion planting—do aromatic plants disrupt host-plant finding by the cabbage root fly and the onion fly more effectively than non-aromatic plants? Entomol Exp Appl 109:183–195
Fleet RR, Young BL (2000) Facultative mutualism between imported fire ants (Solenopsis invicta) and a legume (Senna occidentalis). Southwest Nat 45:289–298
Fullaway DT (1954) Biological control of cactus in Hawaii. J Econ Entomol 47:696–700
Gagnon P, Himmelman JH, Johnson LE (2003) Algal colonization in urchin barrens: defense by association during recruitment of the brown alga Agarum cribrosum. J Exp Mar Biol Ecol 290:179–196
Galloway LF, Fenster CB (2000) Population differentiation in an annual legume: local adaptation. Evolution 54:1173–1181
Gimeno I, Vila M (2002) Recruitment of two Opuntia species invading abandoned olive groves. Acta Oecologica 23:239–246
Hamback PA, Beckerman AP (2003) Herbivory and plant resource competition: a review of two interacting interactions. Oikos 101:26–37
Hamback PA, Agren J, Ericson L (2000) Associational resistance: insect damage to purple loosestrife reduced in thickets of sweet gale. Ecology 81:1784–1794
Hamback PA, Stenberg JA, Ericson L (2006) Asymmetric indirect interactions mediated by a shared parasitoid: connecting species traits and local distribution patterns for two chrysomelid beetles. Oecologia 148:475–481
Hamlin JC (1924) A review of the genus Chelinidea (Hemiptera-Heteroptera) with biological data. Ann Entomol Soc Am 17:193–208
Harmon JP, Ives AR, Losey JE, Olson AC, Rauwald KS (2000) Colemegilla maculata (Coleoptera: Coccinellidae) predation on pea aphids promoted by proximity to dandelions. Oecologia 125:543–548
Hay ME (1986) Associational plant defenses and the maintenance of species-diversity—turning competitors into accomplices. Am Nat 128:617–641
Heil M, McKey D (2003) Protective ant-plant interactions as model systems in ecological and evolutionary research. Annu Rev Ecol Evol S 34:425–453
Hight SD, Carpenter JE (2009) Flight phenology of male Cactoblastis cactorum (Lepidoptera: Pyralidae) at different latitudes in the southeastern United States. Fla Entomol 92:208–216
Holt RD, Lawton JH (1994) The ecological consequences of shared natural enemies. Annu Review Ecol Syst 25:495–520
Hunter WD, Pratt FC, Mitchell JD (1912) The principle cactus insects of the United States. United States Department of Agriculture, Bureau of Entomology, Washington, DC
Janzen DH (1966) Coevolution of mutualism between ants and acacias in Central America. Evolution 20:249–275
Jezorek HA, Stiling PD, Carpenter JE (2010) Targets of an invasive species: oviposition preference and larval performance of Cactoblastis cactorum (Lepidoptera: Pyralidae) on 14 North American opuntioid cacti. Environ Entomol 39:1884–1892
Karban R (1997) Neighbourhood affects a plant’s risk of herbivory and subsequent success. Ecol Entomol 22:433–439
Kelly CA (1986) Extrafloral nectaries: ants, herbivores and fecundity in Cassia fasciculata. Oecologia 69:600–605
Koptur S (1984) Experimental evidence for defense of Inga (Mimosoideae) saplings by ants. Ecology 65:1787–1793
Lach L (2003) Invasive ants: unwanted partners in ant-plant interactions? Ann Mo Bot Gard 90:91–108
Lewis WJ, Stapel JO, Cortesero AM, Takasu K (1998) Understanding how parasitoids balance food and host needs: importance to biological control. Biol Control 11:175–183
Limburg DD, Rosenheim JA (2001) Extrafloral nectar consumption and its influence on survival and development of an omnivorous predator, larval Chrysoperla plorabunda (Neuroptera: Chrysopidae). Environ Entomol 30:595–604
Lobos E, de Cornelli JO (1997) Observations on Cactoblastis cactorum (Berg) as a pest of cactus pear (Opuntia ficus-indica) in Argentina with suggestions on possible control methods. J Prof Assoc Cactus 2:97–102
Mann J (1969) Cactus-feeding insects and mites. Smithsonian Institution bulletin 256, Washington
Mathews CR, Brown MW, Bottrell DG (2007) Leaf extrafloral nectaries enhance biological control of a key economic pest, Grapholita molesta (Lepidoptera: Tortricidae), in peach (Rosales: Rosaceae). Environ Entomol 36:383–389
Milchunas DG, Noy-Meir I (2002) Grazing refuges, external avoidance of herbivory and plant diversity. Oikos 99:113–130
Miller TEX, Legaspi JC, Legaspi B (2010) Experimental test of biotic resistance to an invasive herbivore provided by potential plant mutualists. Biol Invasions 12:3563–3577
Naisbitt T, James EK, Sprent JI (1992) The evolutionary significance of the legume genus chamaecrista, as determined by nodule structure. New Phytol 122:487–492
Ness JH (2003) Contrasting exotic Solenopsis invicta and native Forelius pruinosus ants as mutualists with Catalpa bignonioides, a native plant. Ecol Entomol 28:247–251
Ness JH, Bronstein IL (2004) The effects of invasive ants on prospective ant mutualists. Biol Invasions 6:445–461
Oetting RD (1984) Biology of the cactus scale, Diaspis echinocacti (Bouche) (Homoptera, Diaspididae). Ann Entomol Soc Am 77:89–92
Oliveira PS, Rico-Gray V, Diaz-Castelazo C, Castillo-Guevara C (1999) Interaction between ants, extrafloral nectaries and insect herbivores in Neotropical coastal sand dunes: herbivore deterrence by visiting ants increases fruit set in Opuntia stricta (Cactaceae). Funct Ecol 13:623–631
Pemberton RW, Cordo HA (2001) Potential and risks of biological control of Cactoblastis cactorum (Lepidoptera: Pyralidae) in North America. Fla Entomol 84:513–526
Pettey FW (1947) The biolgical control of prickly pears in South Africa. Department of Agriculture of the Union of South Africa
Pfister CA, Hay ME (1988) Associational plant refuges: convergent patterns in marine and terrestrial communities result from differing mechanisms. Oecologia 77:118–129
Pickett CH, Clark WD (1979) Function of extrafloral nectaries in Opuntia acanthocarpa (Cactaceae). Am J Bot 66:618–625
Porter SD, Savignano DA (1990) Invasion of polygyne fire ants decimates native ants and disrupts arthropod community. Ecology 71:2095–2106
Redman AM, Scriber JM (2000) Competition between the gypsy moth, Lymantria dispar, and the northern tiger swallowtail, Papilio canadensis: interactions mediated by host plant chemistry, pathogens, and parasitoids. Oecologia 125:218–228
Rios RS, Marquis RJ, Flunker JC (2008) Population variation in plant traits associated with ant attraction and herbivory in Chamaecrista fasciculata (Fabaceae). Oecologia 156:577–588
Robbins M, Miller TEX (2009) Patterns of ant activity on Opuntia stricta (Cactaceae), a native host-plant of the invasive cactus moth, Cactoblastis cactorum (Lepidoptera: Pyralidae). Fla Entomol 92:391–393
Robertson HG (1988) Spatial and temporal patterns of predation by ants on eggs of Cactoblastis cactorum. Ecol Entomol 13:207–214
Robertson HG, Hoffmann JH (1989) Mortality and life-tables of Cactoblastis cactorum (Berg) (Lepidoptera: Pyralidae) compared on two host-plant species. B Entomol Res 79:7–17
Root RB (1973) Organization of a plant-arthropod association in simple and diverse habitats: fauna of collards (Brasscia oleracea). Ecol Monogr 43:95–120
Russell EP (1989) Enemies hypothesis: a review of the effect of vegetational diversity on predatory insects and parasitoids. Environ Entomol 18:590–599
Russell FL, Louda SM, Rand TA, Kachman SD (2007) Variation in herbivore-mediated indirect effects of an invasive plant on a native plant. Ecology 88:413–423
Rutter MT, Rausher MD (2004) Natural selection on extrafloral nectar production in Chamaecrista fasciculata: the costs and benefits of a mutualism trait. Evolution 58:2657–2668
Savage AM, Rudgers JA, Whitney KD (2009) Elevated dominance of extrafloral nectary-bearing plants is associated with increased abundances of an invasive ant and reduced native ant richness. Divers Distrib 15:751–761
Settle WH, Wilson LT (1990) Invasion by the variegated leafhopper and biotic interactions: parasitism, competition, and apparent competition. Ecology 71:1461–1470
Sheehan W (1986) Response by specialist and generalist natural enemies to agroecosystem diversification: a selective review. Environ Entomol 15:456–461
Shiojiri K, Takabayashi J, Yano S, Takafuji A (2001) Infochemically mediated tritrophic interaction webs on cabbage plants. Popul Ecol 43:23–29
Shiojiri K, Takabayashi J, Yano S, Takafuji A (2002) Oviposition preferences of herbivores are affected by tritrophic interaction webs. Ecol Lett 5:186–192
Spellman B, Brown MW, Mathews CR (2006) Effect of floral and extrafloral resources on predation of Aphis spiraecola by Harmonia axyridis on apple. Biocontrol 51:715–724
StatSoft, Incorporated (2000) STATISTICA for windows, version 5.5. StatSoft, Incorporated, Tulsa
Stenberg JA, Heijari J, Holopainen JK, Ericson L (2007) Presence of Lythrum salicaria enhances the bodyguard effects of the parasitoid Asecodes mento for Filipendula ulmaria. Oikos 116:482–490
Stiles JH, Jones RH (2001) Top-down control by the red imported fire ant (Solenopsis invicta). Am Midl Nat 146:171–185
Stiling P, Rossi AM, Cattell MV (2003) Associational resistance mediated by natural enemies. Ecol Entomol 28:587–592
Tahvanai JO, Root RB (1972) Influence of vegetational diversity on population ecology of a specialized herbivore, Phyllotreta cruciferae (Coleoptera: Chrysomelidae). Oecologia 10:321–346
Tate CD, Carpenter JE, Bloem S (2007) Influence of radiation dose on the level of F-1 sterility in the cactus moth, Cactoblastis cactorum (Lepidoptera: Pyralidae). Fla Entomol 90:537–544
Thomas CD (1986) Butterfly larvae reduce host plant survival in vicinity of alternative host species. Oecologia 70:113–117
Tilman D (1978) Cherries, ants and tent caterpillars: timing of nectar production in relation to susceptibility of caterpillars to ant predation. Ecology 59:686–692
United States Department of Agriculture, Animal and Plant Health Inspection Service, Plant Protection and Quarantine (2007) Life history of the cactus moth. http://www.aphis.usda.gov/plant_health/plant_pest_info/cactoblastis. Accessed 25 March 2011
Wahl M, Hay ME (1995) Associational resistance and shared doom: effects of epibiosis on herbivory. Oecologia 102:329–340
White JA, Andow DA (2006) Habitat modification contributes to associational resistance between herbivores. Oecologia 148:482–490
White JA, Whitham TG (2000) Associational susceptibility of cottonwood to a box elder herbivore. Ecology 81:1795–1803
Woodruff RE (2009) Cactus weevils, Gerstaeckeria hubbardi (LeConte) and Gerstaeckeria fasciata pierce (Insecta: Coleoptera:Curculionidae). University of Florida Institute of Food and Agricultural Sciences, Department of Entomology and Nematology EENY-82
Zimmermann HG, Bloem S, Klein H (2004) Biology, history, threat, surveillance and control of the cactus moth, Cactoblastis cactorum. International Atomic Energy Agency/Food and Agriculture Organization, Vienna
Acknowledgments
We gratefully thank Susan Drawdy (USDA-ARS, Crop Protection and Management Unit) for help in acquiring C. cactorum eggsticks and pupae, Mark Deyrup (Archbold Biological Station) for ant identification, Michael Gates (USDA-ARS, Systematic Entomology Laboratory) for parasitoid identification, Terry Hingtgen (Florida Department of Environmental Protection, Southwest Division) for his assistance in acquiring the necessary permits for this work, and University of South Florida for providing funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jezorek, H., Stiling, P. & Carpenter, J. Ant predation on an invasive herbivore: can an extrafloral nectar-producing plant provide associational resistance to Opuntia individuals?. Biol Invasions 13, 2261–2273 (2011). https://doi.org/10.1007/s10530-011-0038-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-011-0038-3