Abstract
Main conclusion
This review highlights 50 years of research on the fungal diterpene fusicoccin, during which the molecule went from a tool in plant physiology research to a pharmacological agent in treating animal diseases.
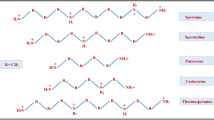
Fusicoccin is a phytotoxic glycosylated diterpene produced by the fungus Phomopsis amygdali, a pathogen of almond and peach plants. Widespread interest in this molecule started when it was discovered that it is capable of causing stomate opening in all higher plants, thereby inducing wilting of leaves. Thereafter, FC became, and still is, a tool in plant physiology, due to its ability to influence a number of fundamental processes, which are dependent on the activation of the plasma membrane H+-ATPase. Molecular studies carried out in the last 20 years clarified details of the mechanism of proton pump stimulation, which involves the fusicoccin-mediated irreversible stabilization of the complex between the H+-ATPase and activatory 14-3-3 proteins. More recently, FC has been shown to influence cellular processes involving 14-3-3 binding to client proteins both in plants and animals. In this review, we report the milestones achieved in more than 50 years of research in plants and highlight recent advances in animals that have allowed this diterpene to be used as a 14-3-3 targeted drug.


Similar content being viewed by others
References
Aducci P, Camoni L, Marra M, Visconti S (2002) From cytosol to organelles: 14-3-3 proteins as multifunctional regulators of plant cell. IUBMB Life 53:49–55
Aitken A, Collinge DB, van Heusden BP, Isobe T, Roseboom PH, Rosenfeld G, Soll J (1992) 14-3-3 proteins: a highly conserved, widespread family of eukaryotic proteins. Trends Biochem Sci 17:498–501
Anders C, Higuchi Y, Koschinsky K, Bartel M, Schumacher B, Thiel P, Nitta H, Preisig-Müller R, Schlichthörl G, Renigunta V, Ohkanda J, Daut J, Kato N, Ottmann C (2013) A semisynthetic fusicoccane stabilizes a protein-protein interaction and enhances the expression of K+ channels at the cell surface. Chem Biol 20:583–593
Ballio A, Chain EB, De Leo P, Erlanger BF, Mauri M, Tonolo A (1964) Fusicoccin: a new wilting toxin produced by Fusicoccum amygdali Del. Nature 203:296
Ballio A, Brufani M, Casinovi CG, Cerrini S, Fedeli W, Pellicciari R, Santurbano B, Vaciago A (1968a) The structure of fusicoccin A. Experientia 24:631–635
Ballio A, Carilli A, Santurbano B, Tuttobello L (1968b) Pilot plant production of fusicoccin. Ann Ist Super Sanita 4:317–332
Barrow KD, Barton DHR, Chain EB, Ohnsorge UVF, Thomas R (1968) The constitution of fusicoccin. Chem Commun 1198–1200
Barrow KD, Barton DHR, Chain EB, Ohnsorge UVF, Thomas R (1971) The constitution of fusicoccin. J Chem Soc C 1265–1273
Baunsgaard L, Fuglsang AT, Jahn T, Korthout HA, de Boer AH, Palmgren MG (1998) The 14-3-3 proteins associate with the plant plasma membrane H(+)-ATPase to generate a fusicoccin binding complex and a fusicoccin responsive system. Plant J 13:661–671
Blatt MR, Clint GM (1989) Mechanisms of fusicoccin action: kinetic modification and inactivation of K + channels in guard cells. Planta 178:509–523
Bunney TD, De Boer AH, Levin M (2003) Fusicoccin signaling reveals 14-3-3 protein function as a novel step in left-right patterning during amphibian embryogenesis. Development 130:4847–4858
Bury M, Andolfi A, Rogister B, Cimmino A, Mégalizzi V, Mathieu V, Feron O, Evidente A, Kiss R (2013a) Fusicoccin A, a phytotoxic carbotricyclic diterpene glucoside of fungal origin, reduces proliferation and invasion of glioblastoma cells by targeting multiple tyrosine kinases. Transl Oncol 6:112–123
Bury M, Girault A, Mégalizzi V, Spiegl-Kreinecker S, Mathieu V, Berger W, Evidente A, Kornienko A, Gailly P, Vandier C, Kiss R (2013b) Ophiobolin A induces paraptosis-like cell death in human glioblastoma cells by decreasing BKCa channel activity. Cell Death Dis 4:e561
Camoni L, Di Lucente C, Visconti S, Aducci P (2011) The phytotoxin fusicoccin promotes platelet aggregation via 14-3-3-glycoprotein Ib-IX-V interaction. Biochem J 436:429–436
Camoni L, Visconti S, Aducci P (2013) The phytotoxin fusicoccin, a selective stabilizer of 14-3-3 interactions? IUBMB Life 65:513–517
Camoni L, Visconti S, Aducci P, Marra M (2018) 14-3-3 proteins in plant hormone signaling: doing several things at once. Front Plant Sci 9:297
Coblitz B, Wu M, Shikano S, Li M (2006) C-terminal binding: an expanded repertoire and function of 14-3-3 proteins. FEBS Lett 580:1531–1535
de Boer AH (2002) Plant 14-3-3 proteins assist ion channels and pumps. Biochem Soc Trans 30:416–421
de Boer AH, de Vries-van Leeuwen IJ (2012) Fusicoccanes: diterpenes with surprising biological functions. Trends Plant Sci 17:360–368
De Michelis MI, Rasi-Caldogno F, Pugliarello MC, Olivari C (1991) Fusicoccin binding to its plasma membrane receptor and the activation of the plasma membrane H+-ATPase. Stimulation of the H+-ATPase in a plasma membrane fraction purified by phase partitioning. Bot Acta 104:265–271
de Vries-van Leeuwen IJ, Kortekaas-Thijssen C, Nzigou Mandouckou JA, Kas S, Evidente A, de Boer AH (2010) Fusicoccin-A selectively induces apoptosis in tumor cells after interferon-alpha priming. Cancer Lett 293:198–206
de Vries-van Leeuwen IJ, da Costa Pereira D, Flach KD, Piersma SR, Haase C, Bier D, Yalcin Z, Michalides R, Feenstra KA, Jiménez CR, de Greef TF, Brunsveld L, Ottmann C, Zwart W, de Boer AH (2013) Interaction of 14-3-3 proteins with the estrogen receptor alpha F domain provides a drug target interface. Proc Natl Acad Sci USA 110:8894–8899
Dohrmann U, Hertel R, Pesci P, Cocucci SM, Marrè E, Randazzo G, Ballio A (1977) Localization of in vitro binding of the fungal toxin fusicoccin to plasma-membrane rich fractions from corn coleoptiles. Plant Sci Lett 9:291–299
Freeman AK, Morrison DK (2011) 14-3-3 Proteins: diverse functions in cell proliferation and cancer progression. Semin Cell Dev Biol 22:681–687
Fu H, Subramanian R, Masters SC (2000) 14-3-3s: structure function and regulation. Annu Rev Pharmacol Toxicol 40:617–647
Fuglsang AT, Visconti S, Drumm K, Jahn T, Stensballe A, Mattei B, Jensen ON, Aducci P, Palmgren MG (1999) Binding of 14-3-3 protein to the plasma membrane H(+)-ATPase AHA2 involves the three C-terminal residues Tyr(946)-Thr-Val and requires phosphorylation of Thr(947). J Biol Chem 274:36774–36780
Fullone MR, Visconti S, Marra M, Fogliano V, Aducci P (1998) Fusicoccin effect on the Interaction between plant 14-3-3 proteins and plasma membrane H -ATPase. J Biol Chem 273(13):7698–7702
Giordanetto F, Schäfer A, Ottmann C (2014) Stabilization of protein-protein interactions by small molecules. Drug Discov Today 19:1812–1821
Graniti A (1962) Phytotoxic action of Fusicoccum amygdali Del. On almond (Prunus amygdali St.). Phytopathol Mediterr 1:182–185
Graniti A (1964) Some phytotoxicity data of fusicoccin A, a toxin produced in vitro by Fusicoccum amygdali Del. Phytopathol Mediterr 3:125–128
Hermeking H (2003) The 14-3-3 cancer connection. Nat Rev Cancer 3:931–943
Honma Y (2002) Cotylenin A—a plant growth regulator as a differentiation-inducing agent against myeloid leukemia. Leuk Lymphoma 43:1169–1178
Honma Y, Akimoto M (2007) Therapeutic strategy using phenotypic modulation of cancer cells by differentiation-inducing agents. Cancer Sci 98:1643–1651
Huber SC, MacKintosh C, Kaiser WM (2002) Metabolic enzymes as targets for 14-3-3 proteins. Plant Mol Biol 50:1053–1063
Jahn T, Fuglsang AT, Olsson A, Brüntrup IM, Collinge DB, Volkmann D, Sommarin M, Palmgren MG, Larsson C (1997) The 14-3-3 protein interacts directly with the C-terminal region of the plant plasma membrane H(+)-ATPase. Plant Cell 9:1805–1814
Kaplan A, Morquette B, Kroner A, Leong S, Madwar C, Sanz R, Banerjee SL, Antel J, Bisson N, David S, Fournier AE (2017a) Small-molecule stabilization of 14-3-3 protein–protein interactions stimulates axon regeneration. Neuron 93:1082–1093.e5
Kaplan A, Ottmann C, Fournier AE (2017b) 14-3-3 adaptor protein-protein interactions as therapeutic targets for CNS diseases. Pharmacol Res 125:114–121
Kent CB, Shimada T, Ferraro GB, Ritter B, Yam PT, McPherson PS, Charron F, Kennedy TE, Fournier AE (2010) 14-3-3 proteins regulate protein kinase A activity to modulate growth cone turning responses. J Neurosci 30:14059–14067
Korthout HA, de Boer AH (1994) A fusicoccin binding protein belongs to the family of 14-3-3 brain protein homologs. Plant Cell 6:1681–1692
Lanfermeijer FC, Prins H (1994) Modulation of H + -atpase activity by fusicoccin in plasma membrane vesicles from Oat (Avena sativa L.) roots (a comparison of modulation by fusicoccin, trypsin, and lysophosphatidylcholine). Plant Physiol 104:1277–1285
Liu D, Bienkowska J, Petosa C, Collier RJ, Fu H, Liddington R (1995) Crystal structure of the zeta isoform of the 14-3-3 protein. Nature 376:191–194
Maki T, Kawamura A, Kato N, Ohkanda J (2013) Chemical ligation of epoxide-containing fusicoccins and peptide fragments guided by 14-3-3 protein. Mol BioSyst 9:940–943
Marra M, Fullone MR, Fogliano V, Pen J, Mattei M, Masi S, Aducci P (1994) The 30-kilodalton protein present in purified fusicoccin receptor preparations is a 14-3-3-like protein. Plant Physiol 106:1497–1501
Marra M, Fogliano V, Zambardi A, Fullone MR, Nasta D, Aducci P (1996) The H+-ATPase purified from maize root plasma membranes retains fusicoccin in vivo activation. FEBS Lett 382:293–296
Marrè E (1979) Fusicoccin: a tool in plant physiology. Annu Rev Plant Physiol 30:273–288
Meyer C, Waldkötter K, Sprenger A, Schlösser UG, Luther M, Weiler EW (1993) Survey of the taxonomic and tissue distribution of microsomal binding sites for the non-host selective fungal phytotoxin, fusicoccin. Z Naturforsch C 48:595–602
Milroy LG, Brunsveld L, Ottmann C (2013) Stabilization and inhibition of protein-protein interactions: the 14-3-3 case study. ACS Chem Biol 8:27–35
Möbius N, Hertweck C (2009) Fungal phytotoxins as mediators of virulence. Curr Opin Plant Biol 12:390–398
Molzan M, Kasper S, Röglin L, Skwarczynska M, Sassa T, Inoue T, Breitenbuecher F, Ohkanda J, Kato N, Schuler M, Ottmann C (2013) Stabilization of physical RAF/14-3-3 interaction by cotylenin A as treatment strategy for RAS mutant cancers. ACS Chem Biol 8:1869–1875
Oecking C, Jaspert N (2009) Plant 14-3-3 proteins catch up with their mammalian orthologs. Curr Opin Plant Biol 12:760–765
Oecking C, Eckerskorn C, Weiler EW (1994) The fusicoccin receptor of plants is a member of the 14-3-3 superfamily of eukaryotic regulatory proteins. FEBS Lett 352:163–166
Oecking C, Piotrowski M, Hagemeier J, Hagemann K (1997) Topology and target interaction of the fusicoccin-binding 14-3-3 homologs of Commelina communis. Plant J 12:441–453
Olivari C, Meanti C, De Michelis MI, Rasi-Caldogno F (1998) Fusicoccin binding to its plasma membrane receptor and the activation of the plasma membrane H(+)-ATPase. IV. Fusicoccin induces the association between the plasma membrane H(+)-ATPase and the fusicoccin receptor. Plant Physiol 116:529–537
Ottmann C, Marco S, Jaspert N, Marcon C, Schauer N, Weyand M, Vandermeeren C, Duby G, Boutry M, Wittinghofer A, Rigaud JL, Oecking C (2007) Structure of a 14-3-3 coordinated hexamer of the plant plasma membrane H+-ATPase by combining X-ray crystallography and electron cryomicroscopy. Mol Cell 25:427–440
Ottmann C, Weyand M, Sassa T, Inoue T, Kato N, Wittinghofer A, Oecking C (2009) A structural rationale for selective stabilization of anti-tumor interactions of 14-3-3 proteins by cotylenin A. J Mol Biol 386:913–919
Ottmann C, Andrei SA, de Vink P, Sijbesma E, Han L, Brunsveld L, Kato N, Higuchi Y (2018) Rationally designed semi-synthetic natural product analogues for stabilization of 14-3-3 protein-protein interactions. Angew Chem Int Ed. https://doi.org/10.1002/anie.201806584
Paiardini A, Aducci P, Cervoni L, Cutruzzolà F, Di Lucente C, Janson G, Pascarella S, Rinaldo S, Visconti S, Camoni L (2014) The phytotoxin fusicoccin differently regulates 14-3-3 proteins association to mode III targets. IUBMB Life 66:52–62
Parvatkar P, Kato N, Uesugi M, Sato S, Ohkanda J (2015) Intracellular generation of a diterpene-peptide conjugate that inhibits 14-3-3-mediated interactions. J Am Chem Soc 137:15624–15627
Pfeilmeier S, Caly DL, Malone JG (2016) Bacterial pathogenesis of plants: future challenges from a microbial perspective: challenges in bacterial molecular plant pathology. Mol Plant Pathol 17:1298–1313
Piotrowski M, Morsomme P, Boutry M, Oecking C (1998) Complementation of the Saccharomyces cerevisiae plasma membrane H+-ATPase by a plant H+-ATPase generates a highly abundant fusicoccin binding site. J Biol Chem 273:30018–30023
Rodolfo C, Rocco M, Cattaneo L, Tartaglia M, Sassi M, Aducci P, Scaloni A, Camoni L, Marra M (2016) ophiobolin A induces autophagy and activates the mitochondrial pathway of apoptosis in human melanoma cells. PLoS One 11:e0167672
Saponaro A, Porro A, Chaves-Sanjuan A, Nardini M, Rauh O, Thiel G, Moroni A (2017) Fusicoccin activates KAT1 channels by stabilizing their interaction with 14-3-3 proteins. Plant Cell 29:2570–2580
Sassa T, Togashi M, Kitaguchi T (1975) The structures of cotylenins A, B, C, D and E. Agric Biol Chem 39:1735–1744
Stevers LM, Lam CV, Leysen SF, Meijer FA, van Scheppingen DS, de Vries RM, Carlile GW, Milroy LG, Thomas DY, Brunsveld L, Ottmann C (2016) Characterization and small-molecule stabilization of the multisite tandem binding between 14-3-3 and the R domain of CFTR. Proc Natl Acad Sci USA 113:E1152–E1161
Sugawara F, Strobel G, Strange RN, Siedow JN, Van Duyne GD, Clardy J (1987) Phytotoxins from the pathogenic fungi Drechslera maydis and Drechslera sorghicola. Proc Natl Acad Sci USA 84:3081–3085
Svennelid F, Olsson A, Piotrowski M, Rosenquist M, Ottman C, Larsson C, Oecking C, Sommarin M (1999) Phosphorylation of Thr-948 at the C terminus of the plasma membrane H(+)-ATPase creates a binding site for the regulatory 14-3-3 protein. Plant Cell 11:2379–2391
Taoka K, Ohki I, Tsuji H, Furuita K, Hayashi K, Yanase T, Yamaguchi M, Nakashima C, Purwestri YA, Tamaki S, Ogaki Y, Shimada C, Nakagawa A, Kojima C, Shimamoto K (2011) 14-3-3 proteins act as intracellular receptors for rice Hd3a florigen. Nature 476:332–335
Turner NC, Graniti A (1969) Fusicoccin: a fungal toxin that opens stomata. Nature 223:1070–1071
Turner NC, Graniti A (1976) Stomatal response of two almond cultivars to fusicoccin. Physiol Plant Pathol 9:175–182
Würtele M, Jelich-Ottmann C, Wittinghofer A, Oecking C (2003) Structural view of a fungal toxin acting on a 14-3-3 regulatory complex. EMBO J 22:987–994
Xiao B, Smerdon SJ, Jones DH, Dodson GG, Soneji Y, Aitken A, Gamblin S (1995) Structure of a 14-3-3 protein and implications for coordination of multiple signalling pathways. Nature 376:188–191
Yaffe MB, Rittinger K, Volinia S, Caron PR, Aitken A, Leffers H, Gamblin SJ, Smerdon SJ, Cantley LC (1997) The structural basis for 14-3-3: phosphopeptide binding specificity. Cell 91:961–971
Yam PT, Kent CB, Morin S, Farmer WT, Alchini R, Lepelletier L, Colman DR, Tessier-Lavigne M, Fournier AE, Charron F (2012) 14-3-3 proteins regulate a cell-intrinsic switch from sonic hedgehog-mediated commissural axon attraction to repulsion after midline crossing. Neuron 76:735–749
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Camoni, L., Visconti, S., Aducci, P. et al. From plant physiology to pharmacology: fusicoccin leaves the leaves. Planta 249, 49–57 (2019). https://doi.org/10.1007/s00425-018-3051-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-018-3051-2