Abstract
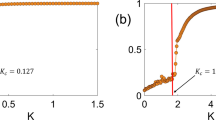
Ant raiding, the process of identifying and returning food to the nest or bivouac, is a fascinating example of collective motion in nature. During such raids ants lay pheromones to form trails for others to find a food source. In this work a coupled PDE/ODE model is introduced to study ant dynamics and pheromone concentration. The key idea is the introduction of two forms of ant dynamics: foraging and returning, each governed by different environmental and social cues. The model accounts for all aspects of the raiding cycle including local collisional interactions, the laying of pheromone along a trail, and the transition from one class of ants to another. Through analysis of an order parameter measuring the orientational order in the system, the model shows self-organization into a collective state consisting of lanes of ants moving in opposite directions as well as the transition back to the individual state once the food source is depleted matching prior experimental results. This indicates that in the absence of direct communication ants naturally form an efficient method for transporting food to the nest/bivouac. The model exhibits a continuous kinetic phase transition in the order parameter as a function of certain system parameters. The associated critical exponents are found, shedding light on the behavior of the system near the transition.










Similar content being viewed by others
References
Amorim P (2014) Modeling ant foraging: a chemotaxis approach with pheromones and trail formation. arXiv:1409.3808
Aranson IS (2013) Collective behavior in out-of-equilibrium colloidal suspensions. C R Phys 14:518–527
Ariel G, Ophir Y, Levi S, Ben-Jacob E, Ayali A (2014) Individual pause-and-go motion is instrumental to the formation and maintenance of swarms of marching locust nymphs. PLOS One 9(7):e101636
Ariel G, Shklarsh A, Kalisman O, Ingham C, Ben-Jacob E (2013) From organized internal traffic to collective navigation of bacterial swarms. New J Phys 15:125019
Bates HW (1863) The naturalist on the river Amazons. Lond Murray 2:350–366
Beckers R, Deneubourg JL, Goss S (1992) Trail laying behavior during food recruitment in the ant Lasius niger (L.). Insectes Soc 39(1):59–72
Boissard E, Degond P, Motsch S (2013) Trail formation based on directed pheromone deposition. J Math Biol 66(6):1267–1301
Buehlmann C, Graham P, Hansson BS, Knaden M (2014) Desert ants locate food by combining high sensitivity to food odors with extensive crosswind runs. Curr Biol 24:960–964
Burger M, Haskovec J, Wolfram M-T (2013) Individual-based and mean-field modelling of direct aggregation. Phy D Nonlinear Phenom 260:145–158
Bonabeau E, Theraulaz G, Deneubourg J-L, Aron S, Camazine S (1997) Self-organization in social insects. TREE 12(5):188–193
Calenbuhr V, Deneubourg J-L (1992) A model for osmotropotactic orientation (I). J Theor Biol 158:343–349
Carillo JA, D’Orsogna MR, Panferov V (2009) Double milling in self-propelled swarms from kinetic theory. Kinet Relat Models 2(2):363–378
Carillo JA, Fornasier M, Toscani G, Vecil F (2010) Particle, kinetic, and hydrodynamic models of swarming. In: Mathematical modeling of collective behavior in socio-economic and life sciences. Birkhäuser Boston, pp 297–336
Colorni A, Dorigo M, Maniezzo V (1991) Distributed optimization by ant colonies. In: Proceedings of the ECAL91 Paris, France. Elsevier, pp 134–142
Couzin ID, Franks NR (2003) Self-organized lane formation and optimized traffic flow in army ants. Proc R Soc Lond B 270:139–146
Czirók A, Barabási A-L, Vicsek T (1999) Collective motion of self-propelled particles: kinetic phase transition in one dimension. Phys Rev Lett 82(1):209–212
D’Orsogna MR, Chuang YL, Bertozzi AL, Chayes LS (2006) Self-propelled particles with soft-core interactions: patterns, stability, and collapse. Phys Rev Lett 96:104302
Degond P, Frouvelle A, Liu J-G (2015) Phase transitions, hysteresis, and hyperbolicity for self-organized alignment. Arch Ration Mech Anal 216:63–115
Dussutour A, Beshers S, Deneubourg J-L, Fourcassie V (2009) Priority rules govern the organization of traffic on foraging trails under crowding conditions in the leaf-cutting ant Atta colombica. J Exper Biol 212:499–505
Erban R, Haskovec J (2012) From individual to collective behaviour of coupled velocity jump processes: a locust example. Kinet Relat Models 5(4):817–842
Escudero C, Yates C, Buhl J, Couzin I, Erban R, Kevrekidis I, Maini P (2010) Ergodic directional switching in mobile insect groups. Phys Rev E 82(1):011926
Franks NR (1985) Reproduction, foraging efficiency and worker polymorphism in army ants. In: Hölldobler B, Lindauer M (eds) Experimental behavioral ecology and socio-biology. Gustav Fischer, Stuttgart, pp 97–107
Garnier S, Combe M, Jost C, Theraulaz G (2013) Do ants need to estimate the geometrical properties of trail bifurcations to find an efficient route? A swarm robotics test bed. PLoS Comput Biol 8(3):e1002903
Garnier S, Guérécheau A, Combe M, Fourcassié V, Theraulaz G (2009) Path selection and foraging efficiency in Argentine ant transport networks. Behav Ecol Sociobiol 63:1167–1179 (2009)
Gotwald WH (1995) Army ants: the biology of social predation. Cornell University Press, Ithaca, NY
Hölldobler B, Wilson EO (1990) The ants. The Belknap Press of Harvard University Press, Cambridge, Mass
Jensen HJ (1998) Self-organized criticality. Cambridge University Press, New York, NY
Johnson K, Rossi LF (2006) A mathematical and experimental study of ant foraging trail dynamics. J Theor Biol 241:360–369
Kaspari M, O’Donnell S (2003) High rates of army ant raids in the Neotropics and implications for ant colony and community structure. Evolut Ecol Res 5:933–939
Keller E, Segel L (1970) Initiation of slide mold aggregation viewed as an instability. J Theor Biol 241:360–369
Keller E, Segel L (1971) Model for chemotaxis. J Theor Biol 26:399–415
Lushi E, Goldstein RE, Shelley MJ (2012) Collective chemotactic dynamics in the presence of self-generated fluid flows. Phys Rev E 86:040902(R)
Ma S-K (1976) Modern theory of critical phenomena. Benjamin, Reading
Martelloni G, Santarlasci A, Bagnoli F, Santini G (2015) Modeling ant battles by means of a diffusion-limited Gillespie algorithm (preprint). arXiv:1503.06094
Müller M, Wehner R (1988) Path integration in desert ants, Cataglyphis fortis. Proc Natl Acad Sci USA 85:5287–5290
Narendra A, Gourmaud S, Zeil J (2013) Mapping the navigational knowledge of individually foraging ants Myrmecia croslandi. Proc R Soc B 280:20130683
Newman MEJ (2005) Power laws, Pareto distributions and Zipf’s law. Contemp Phys 46(5):323–351
Perna A, Granovskiy B, Garnier S, Nicolis SC, Labédan M, Theraulaz G, Fourcassié V, Sumpter DJT (2012) Individual rules for trail pattern formation in Argentine ants (Linepithema humile). PLoS Comput Biol 8(7):e1002592
Powell S, Clark E (2004) Combat between large derived societies: a subterranean army ant established as a predator of mature leaf-cutting ant colonies. Insect Soc 51:342–351
Qi W, Xu Y, Yung K-L, Chen Y (2012) A modified Gay–Berne model for liquid crystal molecular dynamics simulation. Polymer 53:634–639
Ryan SD, Haines BM, Berlyand L, Ziebert F, Aranson IS (2011) Viscosity of bacterial suspensions: hydrodynamic interactions and self-induced noise. Phys Rev E 83:050904(R)
Ryan SD, Berlyand L, Haines BM, Karpeev DA (2013) A kinetic model for semidilute suspensions. SIAM Multiscale Model Simul 11(4):1176–1196
Ryan SD, Sokolov A, Berlyand L, Aranson IS (2013) Correlation properties of collective motion in bacterial suspensions. New J Phys 15:105021
Saintillan D, Shelley MJ (2013) Active suspensions and their nonlinear models. C R Phys 14:497–517
Schmickl T, Thenius R, Crailsheim K (2010) Swarm-intelligent foraging in honeybees: benefits and costs of task-partitioning and environmental fluctuations. Neural Comput Appl 21:251–268
Schneirla TC (1971) Army ants: a study in social organization. Freeman, San Francisco, CA
Schneirla TC (1940) Further studies of the army-ant behavior pattern. Mass organization in the swarm-raiders. J Comp Psychol 29(3):401
Schweitzer F, Lao K, Family F (1997) Active random walkers simulate trunk trail formation by ants. Biosystems 41:153–166
Shklarsh A, Ariel G, Schneidman E, Ben-Jacob E (2011) Smart swarms of bacteria-inspired agents with performance adaptable interactions. PLOS Comput Biol 7(9):e1002177
Sole RV, Bonabeau E, Delgado J, Fernández P, Marín J (2000) Pattern formation and optimization in army ant raids. Artif Life 6:219–226
Stanley HE (1971) Introduction to phase transitions and critical phenomena. Oxford University Press, Oxford
Sumpter DJT, Mann RP, Perna A (2012) The modelling cycle for collective animal behaviour. Interface Focus 2:764–773
Sumpter DJT, Beekman M (2003) From nonlinearity to optimality: pheromone trail foraging by ants. Anim Behav 66:273–280
Tschinkel WR (2006) The fire ants. Harvard University Press, Cambridge, MA
Vicsek T, Czirók A, Ben-Jacob E, Cohen I, Shochet O (1995) Novel type of phase transition in a system of self-driven particles. Phys Rev Lett 75(6):1226
Vicsek T, Zafeiris A (2012) Collective Motion. Phys Rep 517:71–140
Vittori K, Talbot G, Gautrais J, Fourcassié V, Araújo AFR, Theraulaz G (2006) Path efficiency of ant foraging trails in an artificial network. J Theor Biol 239:507–515
Watmough J, Edelstein-Keshet L (1995) A one-dimensional model of trail propagation by army ants. J Math Biol 33:459–476
Watmough J, Edelstein-Keshet L (1995) Modelling the formation of trail netwrosk by foraging ants. J Theor Biol 176:357–371
Wehner R (2003) Desert ant navigation: how miniature brains solve complex tasks. J Comp Physiol A 189:579–588
Wilson EO (1962) Chemical communication among workers of the fire ant Solenopsis saevissima (Fr. Smith) 1. The organization of mass-foraging. Anim Behav 10(1–2):134–138
Winkler M, Falk J, Kinzel W (2015) On the effect of the drive on self-organized criticality. arXiv:1410.5712v3
Wrege PH, Wikelski M, Mandel JT, Rassweiler T, Couzin ID (2005) Antbirds parasitize foraging army ants. Ecology 86(3):555–559
Xue C (2015) Macroscopic equations for bacterial chemotaxis: integration of detailed biochemistry of cell signaling. J Math Biol 70(1–2):1–44
Acknowledgments
Thank you to Paulo Amorim, Gil Ariel, and Magali Tournus for useful discussions. The author gratefully acknowledges support from National Science Foundation Grant DMS-1212046 and advice from X. Zheng (KSU) and P. Palffy-Muhoray (KSU). The work of SR was supported by National Science Foundation Grant DMS-1212046.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix 1: Non-dimensionalization
In order to form a dimensionless problem for the purpose of numerical computations, we must now introduce characteristic scales. A characteristic scale will be denoted by a subscript zero (e.g., \(x_0\)) and a non-dimensional quantity will be denoted with a hat. For example
where the characteristic size of an ant \(x_0 = 1 \text { cm}\) and one option for the characteristic time \(t_0 = 101 s\) is based on the half-life of a food source taken from Amorim (2014). The characteristic diffusion coefficient for pheromone is \(\alpha _0 =x_0^2/t_0 = .01 \text {cm}^2/\text {s}\) and the characteristic concentration of pheromone deposited on a 2D surface is \(c_0 = 1.1\times 10^{-4} \text {g cm}^{-2}\) (both match experimental values from Calenbuhr and Deneubourg 1992; Couzin and Franks 2003).
First, the homogeneous version of the PDE for pheromone concentration (2) becomes
By multiplying through the first equation by \(t_0/c_0\) and the second by \(1/c_0\) we find a non-dimensional equation for the concentration with non-dimensional parameters \(\hat{\alpha },\hat{\gamma }\), and \(\hat{q}\). Once done, we replace the source term responsible for the exponential decay of the pheromone along the trail in dimensionless form
where \(\hat{\gamma } = \gamma t_0\) (\(\gamma \) has units of 1/sec, \(\gamma \approx 1/300s\) in Couzin and Franks 2003). The maximal dimensionless concentration of pheromone deposited is \(\hat{q} = q/c_0\). Next, we proceed to the equations for the foraging ants without the white noise term
By multiplying through the first equation by \(t_0/x_0\) and the second by \(t_0^2/x_0\) we find a non-dimensional equation and add in the dimensionless Gaussian white noise
where \(\hat{U}\) is defined in (1) with dimensionless relative distance \(\hat{r}\) and the dimensionless depth of the potential well \(\hat{\varepsilon } = \varepsilon _0 t_0^2/x^2_0\). Also, \(\hat{\nu } = \nu x_0/t_0\) and \(\hat{d} = dc_0t_0^2/x_0^2\). Similarly for returning ants we find
where \(\hat{\beta } = \beta t_0^2/x_0\) and \(\hat{r} = |\hat{\mathbf{x}}_i-\hat{\mathbf{x}}_c|\). Even though the model (3)–(4) was formulated with dimensional constants, from the dimensional analysis presented in this appendix we recover the necessary dimensions of each of the original quantities if desired. Throughout this work the hats are dropped and all variables are understood as dimensionless.
Appendix 2: Pure pheromone diffusion model
As mentioned in Sect. 5, one can consider a pure diffusion model for the pheromone concentration coupled with the same equations (3)–(4) governing ant dynamics. In this setting the ants only lay pheromone at the food source the moment they become returners. The chemical gradient is formed by diffusion of pheromone in the absence of trail laying. We now introduce the following modified PDE for the pheromone concentration \(c(\mathbf{x}, t)\)
Here M is the total number of visits before a food source is depleted, \(g(\mathbf{x})\) is a constant uniform initial distribution of pheromone, and \(t_j\) is the time that the jth quantity of food is discovered by a forager. This equation models each foraging ant depositing pheromone at the time of each visit. Once the food source is depleted the pheromone concentration naturally decays to zero resulting in the trail disappearing.
This modification results in a small change in the numerical implementation. We now have an explicit analytical solution to the PDE and need to replace (6) with
where
By changing how the ants emit the chemical signal, we achieve an the added advantage of not requiring the computation of a time integral for each foraging ant. One can see from Fig. 11, that the behavior of the ants is the same as in the prior case. The greatest disadvantage is that this approximation to the main model presented is only valid for very short trails where diffusion of pheromone would be sufficient to attract all ants without trail laying.
Sample ant raiding simulations with foragers (purple) and returners (blue) where \(N = 200\). Each arrow represents an individual ant’s orientation \({\varvec{\omega }}_i\). The black circles denote the nest, \(\mathbf{x}_c = (2,0)\), and food source, \(\mathbf{x}_f = (40,0)\). a Initially ants are placed near the nest in non-overlapping positions with random orientation. b Foragers begin to discover the food source and mark it with pheromone, becoming returners. c As the pheromone diffuses more and more foragers detect the scent and begin to follow the trail to the food source. d Once the food source is depleted the trail quickly disappears and the ants return to random foraging. See Online Resource 5 (color figure online)
The main difference in the dynamics from the main model presented (2) is seen when two food sources are present. While locally the motion of each ant may appear similar in each case, the depletion rate of each food source is different. In the case of equidistant food sources, initially both food sources are decreasing at about the same rate, but then the depletion rate of the second food source becomes lower and eventually it is no longer visited as seen by the horizontal portion of the food count function in Fig. 12b. In (2) the ants tended to all raid at the food source which was discovered first and display hardly any trail formation at the second food source until the first was depleted. For food sources at different distances the behavior of both models is similar, the closest food source is essentially depleted first and then the second food source is visited. Thus, ants will use all available foragers to completely deplete the closer quantity of food before moving on. This may provide further evidence of the efficiency in which the ants seek to carry out the raiding process.
Appendix 3: Central nest location
Some additional results are presented where the nest is located at the center of the domain. The purpose of these images is to show that the dynamics and trail formation are essentially the same as in the scenarios presented throughout this work where the nest was closer to one edge of the domain. Figure 13 illustrates the trail formation in time. The only difference is that with the nest in the center it takes longer to attract all the foragers, because some are now farther from any portion of the trail than the previous case. To see the full raid please consult Online Resource 6.
Trail formation with nest in the center. Foragers (purple) and returners (blue) where \(N = 400\). Each arrow represents an individual ant’s orientation \({\varvec{\omega }}_i\). The black circles denote the nest, \(\mathbf{x}_c = (50,0)\), and food source, \(\mathbf{x}_f = (90,0)\). a Once the food source is discovered and phermone is laid along the trail, it takes a greater amount of time for it to diffuse to the ants on the opposite end of the domain. b Eventually all ants join in the raid resulting in a trail with the same form as in Fig. 2. See Online Resource 6 (color figure online)
Rights and permissions
About this article
Cite this article
Ryan, S.D. A model for collective dynamics in ant raids. J. Math. Biol. 72, 1579–1606 (2016). https://doi.org/10.1007/s00285-015-0929-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-015-0929-5
Keywords
- Collective motion
- Phase transition
- Coupled PDE/ODE model
- Ant raiding
- Social insect behavior
- Critical exponents