Abstract
Lateral flow type detection is becoming interesting not only in regions with a poor medical infrastructure but also for practitioners in day-to-day clinical work or for veterinary control in case of possible epidemics. In this work, we describe the first steps of development of a multi-channel strip with potential internal calibration of multiparametric and colorimetric lateral flow assays for the simultaneous detection of the lipopolysaccharides (LPS) of Salmonella typhimurium (S. typhimurium) and Salmonella enteritidis (S. enteritidis). We structured four channels in the nitrocellulose membrane with a Yb:KGW solid-state femtosecond laser (“cold” ablation process) to form distinct tracks of porous material and used gold nanoparticles for the labeling of the antibodies. In addition, calibration curves of the spot intensities of both serovars are presented, and it was shown that no cross reactivity between the different capture antibodies and LPS occurred. Finally, we detected LPS of both Salmonella serovars simultaneously. The color changes (spot intensities of the reaction zones) were evaluated using the open-source image-processing program ImageJ.

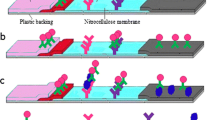
Multiparametric testing, strip A was tested with LPS S. enteritidis ( c=0.01 g/L) and LPS S.typhimurium ( c=0.0001 g/L), strip B with LPS S. enteritidis ( c=0.001 g/L) and LPS S. typhimurium ( c=0.001g/L) and strip C with LPS S. enteritidis (c=0.0001 g/L) and LPS S. typhimurium ( c=0.01 g/L), and read-out



Similar content being viewed by others
References
Birnbaum S, Uden C, Magusson CGM, Nilssson S. Latex-based thin layer immunoaffinity chromatography for quantitaion of protein analytes. Anal Biochem. 1992;206:168–71.
Yeow N, McLiesh H, Guan L, Shen W, Garnier G. Paper-based assay for red blood cell antigen typing by the indirect antiglobulin test. Anal Bioanal Chem. 2016;408:5231–8.
Yager P, Edwards T, Fu E, Helton K, Nelson K, Tam MR, et al. Microfluidic diagnostic technologies for global public health. Nature. 2006;442:412–8.
Jokerts JC, Adkins JA, Bisha B, Mentele MM, Goodrige LD, Henry CS. Development of a paper-based analytical device for colorimetric detection of select foodborne pathogens. Anal Chem. 2012;84:2900–7.
Spyrou EM, Kalogianni DP, Tragoulias SS, Ioannou PC, Christopoulos TK. Digital camera and smartphone as detectors in paper-based chemiluminometric genotyping of single nucleotide polymorphisms. Anal Bioanal Chem. 2016;408:7393–402.
Park TS, Li W, McCracken KE, Yoon JY. Smartphone quantifies Salmonella from paper microfluidics. Lab Chip. 2013;13:4832–40.
Carrilho E, Martinez AW, Whitesides GM. Understanding wax printing: a simple micropatterning process for paper-based microfluidics. Anal Chem. 2009;81:7091–5.
Spicar-Mihalic P, Toley B, Houghtaling J, Liang T, Yager P, Fu E. CO2 laser cutting and ablative etching for the fabrication of paper-based devices. J Micromech Microeng. 2013;23:067003.
Hecht L, Philipp J, Mattern K, Dietzel A, Klages CP. Controlling wettability in paper by atmospheric-pressure microplasma processes to be used in μPAD fabrication. Microfluid Nanofluid. 2016;20(1):1–11.
Dungchai W, Chailapakul O, Henry CS. Use of multiple colorimetric indicators for paper-based microfluidic devices. Anal Chim Acta. 2010;674:227–33.
Kolosova A, De Saeger S, Sibanda L, Verheijen R, Van Peteghem C. Development of a colloidal gold-based lateral-flow immunoassay for the rapid simultaneous detection of zearalenone and deoxynivalenol. Anal Bioanal Chem. 2007;389:2103–7.
Oh YK, Joung HA, Han HS, Suk HJ, Kim MG. A three-line lateral flow assay strip for the measurement of C-reactive protein covering a broad physiological concentration range in human sera. Biosens Bioelectron. 2014;61:285–9.
Hecht L, van Rossum D, Dietzel A. Femtosecond-laser-structured nitrocellulose membranes for multiparameter Point-of-Care tests. Microelectron Eng. 2016;158:52–8.
Holstein CA, Chevalier A, Benett S, Anderson CE, Keniston K, Olsen C, et al. Immobilzing affinity to nitrocellulose: a toolbox for paper-based assay developers. Anal Bioanal Chem. 2016;408:1335–46.
Jaeger S, Ehni M, Eberhardt C, Rolle M, Grathwohl P, Gauglitz G. CCD camera image analysis for mapping solute concentrations in saturated porous media Anal. Bioanal Chem. 2009;395(6):1867–76.
Acknowledgements
This work was funded by the Bundesministerium für Wirtschaft und Energie within the project “Papierbasierte Low Cost Sensorik—Von der Mikrofabrikation bis zur Evaluation”, IGF-Vorhaben, Nr. 18148N/3.
We thank our project partners Tobias Tiemerding and Dr. Albert Still from OFFIS, Oldenburg and Denise van Rossum from Sartorius, Göttingen for the cooperation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors
Additional information
Published in the topical collection celebrating ABCs 16th Anniversary.
Electronic supplementary material
ESM 1
(PDF 406 kb)
Rights and permissions
About this article
Cite this article
Schenk, F., Weber, P., Vogler, J. et al. Development of a paper-based lateral flow immunoassay for simultaneous detection of lipopolysaccharides of Salmonella serovars. Anal Bioanal Chem 410, 863–868 (2018). https://doi.org/10.1007/s00216-017-0643-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0643-9