Abstract
The design of boronic acid sensors for photometric detection of carbohydrates has relied on exploiting differences in the thermodynamic stability of complex formation for molecular recognition. Herein, we introduce a direct method for analysis of sugar alcohols using 3-nitrophenylboronic acid (NPBA) as an electrokinetic probe in capillary electrophoresis (CE). Dynamic complexation of neutral polyols by NPBA during electromigration allows for their simultaneous resolution and UV detection based on formation of an anionic ternary boronate ester complex in phosphate buffer. Unlike conventional boronic acid sensors, thermodynamic and electrokinetic processes in CE allow for improved selectivity for the resolution of sugar alcohol stereoisomers having different vicinal polyol chain lengths even in cases when binding affinity is similar due to differences in their complex mobility. Three complementary approaches were investigated to compare the thermodynamics of polyol chelation with NPBA, namely direct binding assays by CE, UV absorbance spectroscopy and an indirect pK a depression method. Overall, CE offers a convenient platform for characterization of reversible arylboronic acid interactions in free solution while allowing for direct analysis of complex mixtures of neutral/UV-transparent polyols without complicated sample handling.

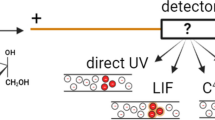
3-nitrophenylboronic acid (NPBA) functions as an electrokinetic probe in capillary electrophoresis for the separation and detection of polyols based on dynamic ternary boronate ester complexation in free solution.




Similar content being viewed by others
Abbreviations
- CE:
-
Capillary electrophoresis
- ∆λ max :
-
Change in peak absorbance wavelength
- ∆pK a :
-
Change in apparent weak acidity equilibrium constant
- EOF:
-
Electroosomotic flow
- f AC :
-
Fraction of tetrahedral boronate ester complex
- K :
-
Conditional formation constant
- μ Aep :
-
Apparent electrophoretic mobility
- μ ep AC :
-
Complex electrophoretic mobility
- NPBA:
-
3-nitrophenylboronic acid
References
Akinterinwa O, Khankal R, Cirino PC (2008) Curr Opin Biotech 19:461–467
Burt, BA (2006) J Am Dent Assoc 137:190–196
Pingle SC, Mishra S, Marcuzzi A, Bhat SG et al (2004) J Biol Chem 279:43157–43167
Mishra R, Seckler R, Bhat R (2005) J Biol Chem 280:15553–15560
Oates PJ (2002) Int Rev Neurobiol 50:325–392
Sim H-J, Jeong J-S, Kwon H-J, Kong TH et al (2009) J Chromatogr B 877:1607–1611
Lee AYW, Chung SSM (1999) FASEB J 13:23–30
Kwang-Hyok S, Ui-Nam P, Sarkar C, Bhadra R, Clin Chim Acta 354: 41–47
Yoshii H, Uchino H, Ohmura C, Watanabe K et al (2001) Diabetes Res Clin Practice 51:115–123
Chalcraft KR, Britz-McKibbin P (2009) Anal Chem 81:307–314
Wamelink MMC, Smith DEC, Jakobs C, Verhoeven NM (2005) J Inherit Metab Dis 28:951–963
Lee J, Chung BC (2006) J Chromatogr B 831:126–131
Kubo E, Urakami T, Fatma N, Akagi Y, Singh DP (2004) Biochem Biophys Res Comm 314:1050–1056
Chung SSM, Ho ECM, Lam KSL, Chung SK (2003) J Am Soc Nephrol 14:S233–S236
Fukuda T, Ishida S, Nakagama T, Koike S, Uchiyama K (2005) Bunseki Kagaku 54:969–973
Anaja HP (2005) Clin Chim Acta 262:1–11
Renner F, Schmitz A, Gehring H (1998) Clin Chem 44:886–887
Schadewaldt P, Hammen H-W, Stolpmann S, Kamalanathan L, Wendel U (2004) J Chromatogr B 801:249–255
Yager C, Wherli S, Segal S (2006) Clin Chim Acta 366:216–224
Ikegami T, Horie K, Saad N, Hosoya K et al (2008) Anal Bioanal Chem 391:2533–2542
Wan ECH, Yu JZ (2007) Environ Sci Technol 41:2459–2466
Cataldi TRI, Campa C, Casella IG, Bufo SA (1999) J Agric Food Chem 47:157–163
Wannet WJB, Hermans JHM, Drift CVD, Camp HJMOD (2000) J Agric Food Chem 48:287–291
Cataldi TRI, Margiotta G, Iasi L, Chio BD et al (2000) Anal Chem 72:3902–3907
Guttman A (1996) Nature 380:461–462
Ma S, Nashabeh W (1999) Anal Chem 71:5185–5192
Szabo Z, Guttman A, Rejtar T, Karger BL (2010) Electrophoresis 31:1–7
Kazarian AA, Hilder EF, Breadmore MC (2008) J Chromatogr A 1200:84–91
Ye J, Baldwin RP (1994) J Chromatogr A 687:141–148
Soga T, Serwe M (2000) Food Chem 69:339–344
Pospisilova M, Polasek M, Safra J, Petriska I (2007) J Chromatogr A 1143:258–263
Soga T, Imaizumi M (2001) Electrophoresis 22:3418–3425
Xu X, Kok WT, Poppe H (1997) J Chromatogr A 786:333–345
Gas B, Kenndler E (2004) Electrophoresis 25:3901–3912
Kaiser C, Segui-Lines G, D'Amaral JC, Britz-McKibbin P (2008) Chem Comm :338–340
James TD, Phillips MD, Shinkai S (2006) In boronic acids in saccharide recognition. The Royal Society of Chemistry, Cambridge, UK
Springsteen G, Wang B (2002) Tetrahedron 58:5291–5300
Bosch LI, Fyles TM, James TD (2004) Tetrahedron 60:11175–11190
Yan J, Springsteen G, Deeter S, Wang B (2004) Tetrahedron 60:11202–11209
Iwatsuki S, Nakajima S, Inamo M, Takagi HD, Ishihara K (2007) Inorg Chem 46:354–356
Zhao J, James TD (2005) J Mater Chem 15:2896–2901
Honda S, Iwase S, Makino A, Fujiwara S (1989) Anal Biochem 63:1541–1547
Hoffstetter-Kuhn S, Paulus A, Gassmann E, Widmer WH (1991) Anal Chem 63:1541–1547
Tsukagoshi K, Hashimoto M, Ichien K, Gen S, Nakajima R (1997) Anal Sci 13:485–487
Tsukagoshi K, Matsumoto K, Ueno F, Noda K et al (2006) J Chromatogr A 1123:106–112
Fenn LS, McLean JA (2008) Chem Comm :5505–5507
Miyamoto C, Suzuki K, Iwatsuki S, Inamo M et al (2008) Inorg Chem 47:1417–1419
Ni W, Fang H, Springsteen G, Wang B (2004) J Org Chem 69:1999–2007
Lim SH, Musto CJ, Park E, Zhong W, Suslick KS (2008) Org Lett 10:4405–4408
Lorand JR, Edwards JO (1959) J Org Chem 24:769–774
Dawber JG, Green SIE (1986) J Chem Soc Faraday Trans 1 82:3407–3413
van Diun M, Peters JA, Kieboom APG, van Bekkum H (1985) Tetrahedron 41:3411–3421
Norrild JC (2001) J Chem Soc Perkin Trans 2:719–726
Kinrade SD, del Nin JW, Schach AS, Sloan TA, Wilson KL, Knight CTG (1999) Science 285:1542–1545
Buchberger W, Cousins SM, Haddad PR (1994) Tr Anal Chem 13:313–319
Collet J, Gareil P (1995) J Chromatogr A 716:115–122
Koy A, Waldhaus A, Hammen H-W, Wendel U, Mayatepek E, Schadewaldt P (2009) Neonatology 95:256–261
Acknowledgments
Financial support for this research was provided by the Natural Science and Engineering Council of Canada. P.B.M. also wishes to acknowledge support in the form of a Japan Society for Promotion of Science—Invited Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fei, F., Britz-McKibbin, P. Direct analysis of polyols using 3-nitrophenylboronic acid in capillary electrophoresis: thermodynamic and electrokinetic principles of molecular recognition. Anal Bioanal Chem 398, 1349–1356 (2010). https://doi.org/10.1007/s00216-010-4038-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-4038-4