Summary
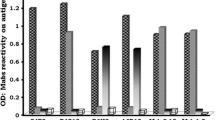
Monoclonal antibodies were prepared against bovine virus diarrhoea virus and hog cholera virus. They were used to test 101 field isolates of ruminant pestivirus in a simple binding assay using an indirect immunoperoxidase label on fixed cell cultures. The monoclonals were divided into three panels: (1) pestivirus group specific, (2) hog cholera specific, (3) selectively reactive with ruminant pestiviruses. The reaction patterns with panel 3 were analyzed by a computer spreadsheet to determine the percentage match with seven reference patterns. Field viruses could be divided into two main groups. Group A consisted of 73 (87%) of the 84 bovine isolates and three (19%) of the 16 ovine, and was reactive with 40% or more of panel 3. Group B showed only limited reactivity with panel 3 and comprised 13/16 (81%) of the ovine (border disease) isolates together with the remaining 11 (13%) bovine viruses.
Similar content being viewed by others
References
Bolin S, Moennig V, Kelso Gourley NE, Ridpath J (1988) Monoclonal antibodies with neutralizing activity segregate isolates of bovine viral diarrhea virus into groups. Arch Virol 99: 117–123
Brownlie J, Clarke MC, Howard CJ (1984) Experimental production of fatal mucosal disease in cattle. Vet Rec 114: 535–536
Brownlie J, Clarke MC, Howard CJ (1987) Clinical and experimental mucosal disease—defining a hypothesis for pathogenesis. In: Harkness JW (ed) Pestivirus infections of ruminants. CEC Agriculture series, EUR 10238, Brussels, pp 147–157
Brownlie J, Clarke MC, Howard CJ, Pocock DH (1987) Pathogenesis and epidemiology of bovine virus diarrhoea virus infection of cattle. Ann Rech Vet 18: 157–166
Goding JW (1986) Monoclonal antibodies: principles and practice, 2nd edn. Academic Press, London, pp 68–76
Hafez SM, Liess B, Frey HR (1976) Studies on the natural occurrence of neutralizing antibodies against six strains of bovine viral diarrhea virus in field sera of cattle. Zentralbl Veterinärmed [B] 23: 669–677
Harkness JW, King AA, Terlecki S, Sands JJ (1977) Border disease of sheep: isolation of the virus in tissue culture and experimental reproduction of the disease. Vet Rec 100: 71–72
Harkness JW, Roeder PL (1988) The comparative biology of classical swine fever. In: Liess B (ed) Classical swine fever and related viral infections. Martinus Nijhoff, Boston, pp 233–288
Holm Jensen M (1981) Detection of antibodies against hog cholera virus and bovine viral diarrhea virus in porcine serum. A comparative examination using CF, PLA, and NPLA assays. Acta Vet Scand 22: 85–98
Horzinek MC (1981) Non-arthropod-borne togaviruses. Academic Press, London, pp 80–83
Howard CJ, Brownlie J, Clarke MC (1987) Comparison by the neutralisation assay of pairs of non-cytopathogenic and cytopathogenic strains of bovine virus diarrhoea virus isolated from cases of mucosal disease. Vet Microbiol 13: 361–369
Kohler G, Howe SC, Milstein C (1976) Fusion between immunoglobulin secreting and non-secreting myeloma cell lines. Eur J Immunol 6: 292–295
Magar R, Minocha HC, Montpetit C, Carman PS, Lecomte J (1988) Typing of cytopathic and noncytopathic bovine viral diarrhea virus reference and Canadian field strains using a neutralizing monoclonal antibody. Can J Vet Res 52: 42–45
Nettleton PF (1987) Pathogenesis and epidemiology of border disease. Ann Rech Vet 18: 147–155
Peters W, Greiser-Wilke I, Moennig V, Liess B (1986) Preliminary serological characterization of bovine viral diarrhoea virus strains using monoclonal antibodies. Vet Microbiol 12: 195–200
Plowright W (1969) Other virus diseases in relation to the JP 15 programme. In: Joint campaign against rinderpest. Proceedings of the 1st technical review meeting, phase IV, Mogadiscio. Organisation of African Unity, Kenya, pp 19–23
Pocock DH, Howard CJ, Clarke MC, Brownlie J (1987) Variation in the intracellular polypeptide profiles from different isolates of bovine virus diarrhoea virus. Arch Virol 94: 43–53
Vantsis JT, Barlow RM, Fraser J, Rennie JC, Mould DL (1976) Experiments in border disease. VIII Propagation and properties of a cytopathic virus. J Comp Pathol 86: 111–120
Westaway EG, Brinton MA, Gaidamovich SYa, Horzinek MC, Igarashi A, Kaariainen L, Lvov DK, Porterfield JS, Russell PK, Trent DW (1985) Togaviridae. Intervirology 24: 125–139
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Edwards, S., Sands, J.J. & Harkness, J.W. The application of monoclonal antibody panels to characterize pestivirus isolates from ruminants in Great Britain. Archives of Virology 102, 197–206 (1988). https://doi.org/10.1007/BF01310825
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01310825