Abstract
Quorum sensing (QS) is a coordination of a group of organisms to exhibit a specific action. It is an acquired social behavior presented to perform either symbiotic or pathogenic activity; however, most of the cases in the absence of QS, the decision to execute certain actions has not been performed. Therefore, QS is also termed as “collective decisions”; it is induced and executed by signaling molecules when the signaling molecule crosses a certain threshold. QS phenomenon is shown by many bacteria and fungi and yeast; recently, viruses also have shown to communicate via QS. In modern research era, study of QS is of most interest for the majority of human healthcare as well as animal health reasons. In general, a key approach perceived is inhibition of QS in case of infections or biofilm formation. Inhibition of QS can reduce the initiation of disease and its severity. Hence, inhibition of QS is of significant interest in human and animal healthcare for developing diagnostic and therapeutic tool. Inhibition of QS is an emerging tool to perform the antimicrobial activity by using targeting agents at either three different levels: production, spread, or acceptance of the signal. The current review primarily emphasizes diverse mechanisms of QS, its inhibition, recent challenges and advances in the field, and its clinical implications in human and animal healthcare.
Similar content being viewed by others
Keywords
1 Introduction
Quorum sensing (QS) is a coordination of a group of organisms to exhibit a specific action (Fuqua et al. 1994). Mechanically, QS is a regulated gene expression by a group of organisms in response to sensing population density. Gene expression is generally dependent on signaling molecule concentration present in the surrounding environment. QS is an acquired social behavior presented to perform a specific group activity like bioluminescence, biofilm formation, and virulence factor expression. However, most of the cases in the absence of quorum sensing, the decision to execute certain actions has not been performed (Boyen et al. 2009). Therefore, QS is also termed as “collective decisions”; it is induced and executed by signaling molecules also known as autoinducers. QS phenomenon is shown by many bacteria and fungi and yeast; recently, viruses also have shown to carry out communication via quorum sensing (Sprague and Winans 2006; Albuquerque and Casadevall 2012; Erez et al. 2017).
Initially, QS was observed in two marine species V. fischeri and V. harveyi by Hastings and Nealson that at a high density of cells, the induction of luciferase enzyme is dependent on the presence of autoinducer (Nealson and Hastings 1979). Communication systems analogous with other organisms were also observed in production of a competence inducing heat-resistant molecule in S. faecalis required for conjugation (Dunny et al. 1978) and in M. xanthus for development of fruiting body (Dworkin and Kaiser 1985). Recently QS, in particular, is of interest because a number of virulent genes are under regulation of QS cascade like cholera caused by V. cholerae (Miller et al. 2002), periodontitis caused by A. actinomycetemcomitans (Fong et al. 2001), banana black rot caused by X. campestris (Barber et al. 1997), and pneumonia caused by K. pneumonia (Russo et al. 2011). However, the clinical significance of bacterial biofilm in diseases was first established in device-related infections (Donlan and Costerton, 2002; Hall-Stoodley and Stoodley 2009). Biofilms share several common features: (i) cells are held together by exopolysaccharides (EPS), (ii) biofilms respond to extracellular signals, (iii) biofilms guard the pathogenic microbes from a wide assortment of environmental stresses, viz., predators, immune system, and antibiotics (Lemon et al., 2008). Bearing in mind the widespread participation of biofilms in infections and diseases in human, biofilms are likely responsible for various infections in veterinary medicine (Clutterbuck et al. 2007). Here, we emphasize the recent understanding on bacterial biofilms, from human and veterinary subjects. This review is deliberated to cover the topics on biofilm formation and strategies for QS inhibition in animal pathogens and to augment consciousness about the potential impact of biofilms on the treatment options.
2 Mechanism of Quorum Sensing
QS is carried out with the diffusible signal molecule which subsequently activates downstream signaling for initiation of gene expression for collective decision. In general, there are three classes of QS. Gram-negative bacteria use acyl homoserine lactone (AHL), while gram-positive bacteria use small peptide molecule as a signaling molecule. AI-2 (LuxS-encoded autoinducer) is common in both gram-positive and gram-negative bacteria.
3 QS in Gram-Negative Bacteria
Up to present, more than 25 gram-negative bacteria species have been identified to communicate via QS. Generally, QS in gram-negative bacteria differs from gram-positive bacteria by two ways, (a) type of QS signaling molecule (also called as autoinducer molecule) and (b) recognition system used by two groups which are different from each other. In gram-negative organisms, QS system is very basic and simple; it consists of signaling molecule and its receptor; at a minimum, it has basic homologs of V. fischeri QS system known as LuxL and LuxR (Miller et al 2001). LuxL gene produces autoinducer molecules in these organisms which are usually N-acetyl-homoserine lactones (AHLS); these are made up of homoserine lactone ring attached to acyl chain of varying length of about 4–18 carbons and variation in saturation of carbon chain (LaSarre and Federle 2013). While LuxR family proteins are a receptor for AHLs, this interaction followed by a regulated response to DNA binding regulates target genes response. In addition to that, the AHL concentration is determined by diffusion extra- and intracellularly once it crosses the critical threshold signaling response is operated (Fig. 7.1) (Tay and Yew 2013).
4 QS in Gram-Positive Bacteria
Density-dependent regulation of certain responses and cell-to-cell communication are also established in gram-positive organisms. They instead of AHLs use secretary peptides as autoinducers. Precursor peptide chain is cleaved to produce a signal, and it is transported extracellularly via ABC transporters (LaSarre and Federle 2013). Peptides before extracellular transfer undergo different posttranslational processing or cyclization. Usually, detection of this peptide occurs by the extracellular receptor, histidine kinase, which autophosphorylates and transfers phosphate to response regulator molecule, which possesses DNA-binding capacity upon phosphorylation and finally regulates the response (Fig. 7.1).
5 QS in Both Gram-Positive and Gram-Negative Bacteria
Autoinducer-1 is another QS signal produced by many gram-positive and gram-negative species. Chemically, it is furanosyl borate diester part of the two-component system. It is produced by luxP and recognized by a protein having regulatory activity, LuxQ, while signal transduction is carried out by sensor kinase (LaSarre and Federle 2013). Strikingly, this QS system is common for luciferase operon expression observed in V. harveyi and S. enterica. The autoinducer AI-2 could serve as a “universal signal” for interspecies communication (Xavier and Bassler 2003).
6 Implications of QS in Human and Veterinary Pathogens
P. aeruginosa, A. baumannii, and K. pneumonia and Enterobacter spp. are well-known highly pathogenic bacteria and are the leading cause of several human nosocomial, urinary tract, respiratory tract, blood, and burn or wound infections (Lister et al. 2009). In Enterobacter spp., E. coli is a normal flora of GI tract where sometimes it acts as opportunistic pathogen also known for respiratory and urinary tract infection. Enterobacter spp. possess multiple mechanisms of QS, commonly used AHLs based in E. coli (Pinto et al. 2007; dos Reis Ponce et al. 2012), GS1-S-SdiA in E. cloacae (Shankar et al. 2013), and EAL-(SdiA-AL2) interaction, AI3. This biofilm-forming Enterobacter spp. always follows some communication or interaction among them (dos Reis Ponce et al. 2012) with P. aeruginosa. Moreover, virulent bacteria K. pneumonia enhances its virulence properties via AI-2, AHL, and siderophore-related QS molecule production and successfully mounts the virulence and pathogenesis (Zhu et al. 2011; Russo et al. 2011). S. aureus, S. pneumonia, P. pseudintermedius, and Salmonella are the highly pathogenic and virulent strains in veterinary therapeutics and cause various infections in rabbits, cows, dogs, cats, etc. S. aureus in rabbit is responsible for skin infection, mastitis, and internal abscessation (Vancraeynest et al. 2006), while in bovine, it causes skin and mammary gland infection (Buzzola et al. 2007). P. pseudintermedius causes mastitis, otitis, dermatitis, and hemorrhagic pneumonia, while Salmonella mostly causes gastrointestinal complications. Accessory control regulator has highly studied QS among gram-positive organisms (Novick 2003), and it is a regulator of many virulent genes, such as hemolysins, enterotoxins, different proteins, and toxins. Strikingly, Pseudomonas sp. regulates its virulence factor by Las and Rh1QS mechanism (Girard and Bloemberg 2008). However, Salmonella QS system is composed of LuxR homolog and SdiA (Walters and Sperandio 2006).
Antibiotic resistance (AR) is a devastating problem if we take a closer look at the response of virulent organisms to existing antibiotics; the rate at which these microbes are developing resistant is quite alarming (Dellit et al. 2007). It is pertinent that the selection pressures induced due to the incorporation of drugs into the environment while targeting, these drugs are deemed as vital targets to bacterial metabolism. AR genes can spread rapidly to biofilms, may perhaps endure longer in the biofilms, and act as reservoirs for AR genes. Consequently, the exploitation of antibiotics to treat biofilm-associated bacterial infections are expected to hasten the expansion and spread of AR in bacteria (Ceri et al. 2010). This practice has lost effectively of many potential drugs; however, total numbers of available drugs for the treatment of such pathogenic organisms are limited now (Fernandes 2006; Dellit et al. 2007). However, the field of therapeutics is a burning need for designing novel antibacterial therapies with the aim to impair bacterial pathogenesis while preventing the generation of new drug-resistant strains. QS inhibition-based approach can be one of such antivirulence or antipathogenesis approach rather than providing bactericidal effects. The current review article, however, summarizes the current knowledge about the development of new strategies and anti-quorum sensing approaches for the treatment of human and veterinary diseases.
QS systems are conscientious for the expression of many bacterial virulence factors; hence, disruption or blocking of density-dependent communication between virulent pathogens is an effective way to interrupt cooperatively. Knowledge earned from last 45 years about the social behavior of pathogenic organisms during induction and progression of the disease is used to inhibit QS at different levels (LaSarre and Federle 2013). Additionally, QS inhibition unlikely affects the crucial processes of bacterial growth. Hence, disruption of QS, therefore, does not exert antibiotic-associated selection pressure (Rasko and Sperandio 2010). If QS is an essential task in bacterial life cycle, then there may be possibilities of development of anti-QS drug-resistant strains, keeping mind this could help us for promoting long-term efficacy of anti-QS drugs (LaSarre and Federle 2013). It is noteworthy that for some organisms, living in mixed consortia would provide protection; if these efforts are blocked, theoretically bacteria would be unable to mount an attack; on the other hand, bacteria become susceptible for any given antibiotic range. Hence, the study of QS in pathogenic organisms and the discovery of new anti-QS drugs will undeniably enhance the availability of drugs useful for human and veterinary treatment.
7 QS Inhibition in In Vitro
Ideally, QS inhibitor should be small in size, chemically stable, narrow range of action, and nontoxic to eukaryotes. There are several strategies to prevent and inhibit biofilm formation. These strategies include the prevention of microbial attachment, prevention of microbial growth, disruption of cell-to-cell communication, inhibition of matrix synthesis, and disintegration of the biofilm matrix (Anderson and O’Toole 2008).
-
(a)
Inhibition of QS Inducer Production
Quite a few studies have been performed yet; therefore, the field is open for exploration. Naturally occurring quorum sensing inhibitor (QSI) is in large number but due to low amount availability and associated toxic effects made us to search chemically synthesized QSI. QS signal analogs like 3-oxy-acyl carrier protein, butyryl-S-adenosylmethionine, L/D-S-adenosylhomocysteine, and sinefungin have the ability to block AHL synthase production. But in vivo studies have not been performed as they also affect amino acid and fatty acid biosynthesis. A molecule targeting AHL synthase has been recently studied by Chung et al. (2011).
Another potential target is Methylthioadenosine/s-adenosylhomocysteine nucleosidase enzyme (MTAN). This enzyme is conserved in bacterial species only and involved in the biosynthesis of AI-1 and AI-2. Analog of MTA could be used without affecting eukaryotic cellular metabolism. Furthermore plethora of studies have reported the ability of sulfur-free and sulfur-containing transition state analogs as potent inhibitors for E. coli MTAN and can be further studied for drug design to arise effective analogs (Scutera et al. 2014). Anthranilate is a precursor molecule for pseudomonas QS signal known as pseudomonas quinolone signal (PQS). Calfee et al. (2001) studied methyl anthranilate effectively which inhibits the production of PQS. However, P. aeruginosa PQS can activate the genes for both LasB elastase (lasB) and the C4-HSLsynthase (rhlI) in P. aeruginosa. This signal was also shown to be part of the quorum-sensing hierarchy in P. aeruginosa. N-(3-oxododecanoyl)-L-homoserine lactone and N-butyryl-L-homoserine lactone acts as intercellular signals. These two signaling systems can be effectively regulated by decreasing transcriptional activation of las and pqs genes (Zhou et al. 2013); together, these therapies may provide a new remedy for many Pseudomonas infections.
-
1.
Targeting QS Signaling Molecule
Decreasing concentration of QS signaling molecule will automatically shut down QS and connected expression of virulent genes thereby pathogenesis. As QS signal is secreted out of the cells, they are very easy to target. Inactivation of the signal is achieved by various methods. Commonly, enzymatic degradation or antibody-mediated depletion is popular and easy for study.
-
(a)
Enzymes
Broadly QSI enzymes are categorized into two types: oxidoreductases which reduce carbonyl to a hydroxyl group and AL-lactonase, AHL acylase, and paraoxonase which cleave the AHLs. Till date, three oxidoreductases have been identified. CYP102A1 from Bacillus megaterium can oxidize both acyl homoserine lactone and acyl homoserine. W2 from Rhodococcus erythropolis is the enzyme having a dual activity of oxidoreduction along with amylase activity (Uroz et al. 2005; Chowdhary et al. 2007). Recently identified BpiB09 through metagenomics screening can inactivate 3-oxo-c12-homoserine lactone (Bijtenhoorn et al. 2011). Practically, 20 AHL lactonases are known and can be used for disruption of QS signaling molecule. A good example is PvdQ enzyme belonging to nucleotide hydrolase super family. This enzyme produced by P. aeruginosa PA01 strain explains it might be a strategy to inhibit its own QS. Overexpression studies showed inhibitory activity (Papaioannou et al. 2009) and successive production of stable powder formulation which is further used in treatment purpose (Yang et al. 2005).
-
(b)
Antibodies
QS signaling molecules are relatively smaller in size; therefore, eukaryote is unable to mount an antibody response; in return, these molecules can elicit apoptosis and modulation of NF-kB activity. In an earlier work on an antibody generation against synthetic 3-oxo-AHL, RS2 was found effective on long side chain AHL but not on short chain AHLs. Vaccination strategy for AHL-carrier conjugate in mice model for lung infection showed a positive result with increased progression of disease (Miyairi et al. 2006). Subsequently, the study by Park et al. highlighted generation of the immune response against a lethal challenge of S. aureus with the usage of a monoclonal antibody against (AIP)-4. This encouraged the descending investigators to generate the monoclonal antibody. Palliyil et al. generated a monoclonal antibody against P. aeruginosa by phage display library construct preparation and screened with 1000 times more affinity (Palliyil et al. 2014).
-
2.
Targeting Signal Detection/Receptor
Targeting signal detection/receptor is done via either of two methods.
-
(a)
Natural Analogs
Natural compounds are very complex in nature with differential actions, which sometimes is difficult to design in the lab. The only difficulty with natural compounds is to get in bulk (LaSarre and Federle 2013). In gram-negative organisms, widely used reporter strain for measuring inhibitory activity is C. violaceum and V. fischeri (Rasmussen et al. 2005; McClean et al. 1997).
-
(b)
Furanones
Brominated furanones were the first recognized small natural molecules as quorum sensing inhibitors (QSI); other forms include halogenated and chlorinated furanones, with the only concern being toxicity to the hosts. They have been mainly isolated from diverse sources including plants, marine samples, algae, fungi, bacteria, etc. proven to have inhibitory activity against gram-negative and gram-positive organisms. The following furanone molecules, C-56 (Rasch et al. 2004; Wu et al. 2004), C-30 (Hentzer et al. (2002); DC-917 inhibits growth of mouse lung carcinoma), C-2 (Ren et al. 2004) are extensively studied in pathogenic organisms, viz., P. aeruginosa, V. harveyi, and V. campbellii. In vivo studies in a mouse model showed no any toxic effects; but still, these compounds need to undergo clinical trials.
-
(c)
Compounds Other Than Furanones
Due to the aquatic environment, these compounds generally occur in low concentration from sponges, microalgae, coral-associated bacteria, and cyanobacteria (LaSarre and Federle 2013). They can act on AHL as well as on AL2-based organisms. V. harveyi, C. violaceum, and E. coli QS can effectively inhibit without hampering growth (Skindersoe et al. 2008; Teasdale et al. 2009; Dobretsov et al. 2010). Surprisingly, these compounds also have shown to inhibit the inflammatory responses of macrophages via inhibiting NF-kB signaling. Halogenated derivatives showed potent activity than original compound (Kravchenko et al. 2008) (Table 7.1).
-
(d)
Peptide-Based Inhibitors
These compounds are produced by a diverse range of microorganisms including Actinomycetes. Siamycin, diketopiperazines, cyclo[L-Tyr-(L or D)-Pro], and cyclo(L-Phe-L-Pro) are the cyclic peptides identified to have anti-QS activity; they have been tested for inhibition of gelatinase, secretary protease, and arginase production from E. faecalis and arginase-producing organisms. These natural compounds need to be isolated and studied for the mechanism of action; it may open new avenues for the study of QS inhibition and its use as a diagnostic tool for human and animal diseases. Recently, one group has tested the anti-QS effectiveness of essential oils and a combination of it. Results showed appreciable anti-QS and biosurfactant activity; therefore, employing such compounds is practical in biofilm-forming pathogens as well (Mukherji and Prabhune 2014). Use of these quenching agents is done successfully in controlling some diseases. Harmful fish pathogen A. hydrophila infection is reduced upon carp feeding on AiiA or recombinant Bacillus which can able to produce AiiA (Chen et al. 2010). But still, for approved medicinal use, anti-QS medicines need to undergo extensive clinical trials.
-
(e)
Fatty Acid-Based Inhibitors
Numerous examples of fatty acid-based QS inhibitors capable of meddling with biofilm formation have been reported in recent times. Davies and Marques (2009) depicted a small messenger fatty acid molecule produced by P. aeruginosa, cis-2-decenoic acid, competent of inhibiting biofilm development. One of the marine natural product derivative bromoageliferin also named TAGE (transbromoageliferin analog) depicted antibiofilm activity against P. aeruginosa (Huigens et al. 2008; Truchado et al. 2009) evidenced that chestnut honey and its aqueous extract were evidenced as QS inhibitors. These two compounds also appreciably reduced the biofilm formation in Y. enterocolitica and A. hydrophila. The cathelicidin LL-37 was revealed to hinder F. novicida biofilm formation at subinhibitory concentrations (Amer et al. 2010).
-
(f)
Synthetic Compounds
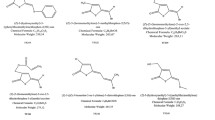
Approach to synthetic compounds is a best-studied strategy for inhibiting the QS signals perceived by the bacteria. The synthetic analogs and antagonists act by blocking or destructing the receptor–ligands interaction and downstream signaling. Computer-aided drug designing screening of small molecules also increased the range of QS systems agonists and antagonist which destructs the QS signaling. Agonists and antagonists of AHL receptors, AL-2 antagonists, and peptide analogs are currently in use. Synthetic AHL analogs are synthesized from natural AHL molecules by modifying the length, saturations, and oxidation states of natural AHL compounds. Among different types of QS molecules, AHL receptors like Lux-R, LasR, and TraR interactions are most studied with synthetic analogs (LaSarre and Federle 2013) (Table 7.1).
8 Future Perspectives and Conclusions
Research on biofilms is therefore an area of extreme curiosity (Haussler and Parsek 2010) and thus gained appreciation in animal and public health. Researchers now have access to a wide battery of techniques together with 3D imaging, advanced fluorescent stains, confocal laser scanning microscopy (CLSM), and molecular reporter gene technology to research on biofilms. During the recent developments, next-generation antimicrobial compounds, are of serious concern ought to the synergies between antibiotics and molecules (e.g., enzymes, biocides, surfactants, metals, or QS inhibitors) for the treatment of biofilms (Ceri et al. 2010). There is an impending prospective for augmented resistance to antibiotics, disinfectants, and host immune response. This interferes with the effective treatment of animals and human subjects. The persistence of antibiotic resistance genes within biofilms is an additional aspect that isn’t a surprise, for the reason that it has a probable impact on animal and public health equally. Further research is also obligatory to expand successful disinfection protocols to get rid of biofilms from the human and veterinary environments, since biofilms can serve as a reservoir for any given infectious agents (Clutterbuck et al. 2007). Ever since the technological advancements increased the rate of discovery of new QS signaling organism, molecules, receptors, and understanding of mechanism behind it. But the field is still evolving, while the targeting QS restricts the virulence properties of pathogenic organisms without affecting growth; study of anti-QS strategies is of much interest. Use of many anti-QS approaches in different gram-negative and gram-positive reporter strains showed effective results in in vitro and in few in vitro case studies. The field needs further computational studies to develop better compounds and clinical studies for testing the effectiveness of anti-QS compounds for disease treatment. In future disease, therapy will be common to use anti-QS drugs in combination to microbicidal drugs in veterinary and human medicine world.
References
Albuquerque P, Casadevall A (2012) Quorum sensing in fungi–a review. Med Mycol 50(4):337–345
Amer LS, Bishop BM, van Hoek ML (2010) Antimicrobial and antibiofilm activity of cathelicidins and short, synthetic peptides against Francisella. Biochem Biophys Res Commun 396:246
Anderson GG, O’toole GA (2008) Innate and induced resistance mechanisms of bacterial biofilms. In: Bacterial biofilms. Springer, Berlin, Heidelberg, pp 85–105
Barber CE et al (1997) A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol Microbiol 24(3):555–566
Bijtenhoorn P et al (2011) A novel metagenomic short-chain dehydrogenase/reductase attenuates Pseudomonas aeruginosa biofilm formation and virulence on Caenorhabditis elegans. PLoS One 6(10):e26278
Boyen F et al (2009) Quorum sensing in veterinary pathogens: mechanisms, clinical importance and future perspectives. Vet Microbiol 135(3–4):187–195
Buzzola FR et al (2007) Differential abilities of capsulated and noncapsulated Staphylococcus aureus isolates from diverse agr groups to invade mammary epithelial cells. Infect Immun 75(2):886–891
Calfee MW, Coleman JP, Pesci EC (2001) Interference with Pseudomonas quinolone signal synthesis inhibits virulence factor expression by Pseudomonas aeruginosa. Proc Natl Acad Sci 98(20):11633–11637
Ceri H, Olson ME, Turner RJ (2010) Needed, new paradigms in antibiotic development. Expert Opin Pharmacother 11:1233–1237
Chen R et al (2010) High yield expression of an AHL-lactonase from Bacillus sp. B546 in Pichia pastoris and its application to reduce Aeromonas hydrophila mortality in aquaculture. Microb Cell Factories 9(1):39
Chowdhary PK et al (2007) Bacillus megaterium CYP102A1 oxidation of acyl homoserine lactones and acyl homoserines. Biochemistry 46(50):14429–14437
Chung J et al (2011) Small-molecule inhibitor binding to an N-acyl-homoserine lactone synthase. Proc Natl Acad Sci 108(29):12089–12094
Clutterbuck AL, Woods EJ, Knottenbelt DC, Clegg PD, Cochrane CA, Percival SL (2007) Biofilms and their relevance to veterinary medicine. Vet Microbiol 121:1–17
Davies DG, Marques CNH (2009) A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J Bacteriol 191:1393–1403
Dellit TH et al (2007) Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 44(2):159–177
Dobretsov S et al (2010) Malyngolide from the cyanobacterium Lyngbya majuscula interferes with quorum sensing circuitry. Environ Microbiol Rep 2(6):739–744
Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193
dos Reis Ponce A et al (2012) AiiA quorum-sensing quenching controls proteolytic activity and biofilm formation by Enterobacter cloacae. Curr Microbiol 65(6):758–763
Dunny GM, Brown BL, Clewell DB (1978) Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc Natl Acad Sci 75(7):3479–3483
Dworkin M, Kaiser D (1985) Cell interactions in myxobacterial growth and development. Science 230(4721):18–24
Erez Z et al (2017) Communication between viruses guides lysis–lysogeny decisions. Nature 541(7638):488
Fernandes P (2006) Antibacterial discovery and development—the failure of success? Nat Biotechnol 24(12):1497
Fong KP et al (2001) Intra- and interspecies regulation of gene expression by Actinobacillus actinomycetemcomitans LuxS. Infect Immun 69(12):7625–7634
Fuqua WC, Winans SC, Peter Greenberg E (1994) Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol 176(2):269
Girard G, Bloemberg GV (2008) Central role of quorum sensing in regulating the production of pathogenicity factors in Pseudomonas aeruginosa. Future Microbiol 3:97–106
Hall-Stoodley L, Stoodley P (2009) Evolving concepts in biofilm infections. Cell Microbiol 11:1034–1043
Haussler S, Parsek MR (2010) Biofilms 2009: new perspectives at the heart of surface-associated microbial communities. J Bacteriol 192:2941–2949
Hentzer M et al (2002) Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 148(1):87–102
Huigens RW III, Ma L, Gambino C, Moeller PDR, Basso A, Cavanagh J, Wozniak DJ, Melander C (2008) Control of bacterial biofilms with marine alkaloid derivatives. Mol BioSyst 4:614–621
Kalia VC (2013) Quorum sensing inhibitors: an overview. Biotechnol Adv 31(2):224–245
Kravchenko VV et al (2008) Modulation of gene expression via disruption of NF-κB signaling by a bacterial small molecule. Science 321(5886):259–263
LaSarre B, Federle MJ (2013) Exploiting quorum sensing to confuse bacterial pathogens. Microbiol Mol Biol Rev 77(1):73–111
Lemon KP, Earl AM, Vlamakis HC, Aguilar C, Kolter R (2008) Biofilm development with an emphasis on Bacillus subtilis. Curr Top Microbiol Immunol 322:1–16
Lister PD, Wolter DJ, Hanson ND (2009) Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev 22(4):582–610
McClean KH et al (1997) Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143(12):3703–3711
Miller MB et al (2002) Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell 110(3):303–314
Miyairi S et al (2006) Immunization with 3-oxododecanoyl-L-homoserine lactone–protein conjugate protects mice from lethal Pseudomonas aeruginosa lung infection. J Med Microbiol 55(10):1381–1387
Mukherji R, Prabhune A (2014) Novel glycolipids synthesized using plant essential oils and their application in quorum sensing inhibition and as antibiofilm agents. Sci World J 2014:890709
Nealson KH, Hastings JW (1979) Bacterial bioluminescence: its control and ecological significance. Microbiol Rev 43(4):496
Novick RP (2003) Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol 48(6):1429–1449
Palliyil S et al (2014) High-sensitivity monoclonal antibodies specific for homoserine lactones protect mice from lethal Pseudomonas aeruginosa infections. Appl Environ Microbiol 80(2):462–469
Papaioannou E et al (2009) Quorum-quenching acylase reduces the virulence of Pseudomonas aeruginosa in a Caenorhabditis elegans infection model. Antimicrob Agents Chemother 53(11):4891–4897
Pinto UM et al (2007) Detection of acylated homoserine lactones in gram-negative proteolytic psychrotrophic bacteria isolated from cooled raw milk. Food Control 18(10):1322–1327
Rasch M et al (2004) An inhibitor of bacterial quorum sensing reduces mortalities caused by vibriosis in rainbow trout (Oncorhynchus mykiss, Walbaum). Syst Appl Microbiol 27(3):350–359
Rasko DA, Sperandio V (2010) Anti-virulence strategies to combat bacteria-mediated disease. Nat Rev Drug Discov 9(2):117
Rasmussen TB et al (2005) Screening for quorum-sensing inhibitors (QSI) by use of a novel genetic system, the QSI selector. J Bacteriol 187(5):1799–1814
Ren D et al (2004) Differential gene expression shows natural brominated furanones interfere with the autoinducer-2 bacterial signaling system of Escherichia coli. Biotechnol Bioeng 88(5):630–642
Russo TA et al (2011) Hypervirulent K. pneumoniae secretes more and more active iron-acquisition molecules than “classical” K. pneumoniae thereby enhancing its virulence. PLoS One 6(10):e26734
Scutera S, Zucca M, Savoia D (2014) Novel approaches for the design and discovery of quorum-sensing inhibitors. Expert Opin Drug Discovery 9(4):353–366
Shankar M et al (2013) Inactivation of the transcriptional regulator-encoding gene sdiA enhances rice root colonization and biofilm formation in Enterobacter cloacae GS1. J Bacteriol 195(1):39–45
Skindersoe ME et al (2008) Quorum sensing antagonism from marine organisms. Mar Biotechnol 10(1):56–63
Sprague GF, Winans SC (2006) Eukaryotes learn how to count: quorum sensing by yeast. Genes Dev 20(9):1045–1049
Tay S, Yew W (2013) Development of quorum-based anti-virulence therapeutics targeting Gram-negative bacterial pathogens. Int J Mol Sci 14(8):16570–16599
Teasdale ME et al (2009) Secondary metabolites produced by the marine bacterium Halobacillus salinus that inhibit quorum sensing-controlled phenotypes in gram-negative bacteria. Appl Environ Microbiol 75(3):567–572
Truchado P, Gil-Izquierdo A, Tomas-Barberan F, Allende A (2009) Inhibition by chestnut honey of N-acyl-L-homoserine lactones and biofilm formation in Erwinia carotovora, Yersinia enterocolitica, and Aeromonas hydrophila. J Agric Food Chem 57:11186–11193
Uroz S et al (2005) N-Acylhomoserine lactone quorum-sensing molecules are modified and degraded by Rhodococcus erythropolis W2 by both amidolytic and novel oxidoreductase activities. Microbiology 151(10):3313–3322
Vancraeynest D et al (2006) International dissemination of a high virulence rabbit Staphylococcus aureus clone. J Veterinary Med Ser B 53(9):418–422
Walters M, Sperandio V (2006) Quorum sensing in Escherichia coli and Salmonella. Int J Med Microbiol 296(2–3):125–131
Wu H et al (2004) Synthetic furanones inhibit quorum-sensing and enhance bacterial clearance in Pseudomonas aeruginosa lung infection in mice. J Antimicrob Chemother 53(6):1054–1061
Xavier KB, Bassler BL (2003) LuxS quorum sensing: more than just a numbers game. Curr Opin Microbiol 6(2):191–197
Yang F et al (2005) Quorum quenching enzyme activity is widely conserved in the sera of mammalian species. FEBS Lett 579(17):3713–3717
Zhou L et al (2013) Eugenol inhibits quorum sensing at sub-inhibitory concentrations. Biotechnol Lett 35(4):631–637
Zhu H et al (2011) A luxS-dependent transcript profile of cell-to-cell communication in Klebsiella pneumoniae. Mol BioSyst 7(11):3164–3168
Acknowledgments
The authors are grateful to Krishna University, Machilipatnam, NIV Pune and NCCS Pune for the support extended.
Conflict of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Bhukya, P.L., Nawadkar, R., Bramhachari, P.V., Sheela, G.M. (2019). Significance of Quorum Sensing and Biofilm Formation in Medicine and Veterinary Sciences. In: Bramhachari, P. (eds) Implication of Quorum Sensing and Biofilm Formation in Medicine, Agriculture and Food Industry . Springer, Singapore. https://doi.org/10.1007/978-981-32-9409-7_7
Download citation
DOI: https://doi.org/10.1007/978-981-32-9409-7_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-32-9408-0
Online ISBN: 978-981-32-9409-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)