Abstract
So far we have been concerned with the combustion of gaseous fuels. However, solid (coal, wood, agricultural residues, or metals) and liquid (diesel, kerosene, ethanol, etc.) fuels are routinely used in a variety of applications. Such fuels burn at a relatively slow flux rate, expressed in kg/m\(^{2}\)-s. To achieve high heat release rates (J/s), it becomes necessary to increase the surface area of the fuel. This is achieved by atomizing liquid fuels and by pulverizing solid fuels.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Notes
- 1.
Very fine coal particles are, in fact, carried away upward and burnt in suspension.
- 2.
The bed also assumes many properties of a real fluid. For example, when tilted, the free surface of the bed remains horizontal.
- 3.
Largely, to prevent this carryover burning and the associated loss of unburned carbon, an alternative concept of a circulating fluidized bed is now practiced. In this, the air velocity through the distributor is high enough to physically transport the particles upward to the full chamber height, as shown in Fig. 10.3(b). The particles are then returned via the downward leg of a cyclone separator. This type of particle burning achieves high mixing rates and there is no clearly defined bed. Also, there are no embedded tubes in the combustion zone. The burned gases are led directly to the superheater tubes and the convection section. The circulating fluidized bed is particularly suitable for high-ash-content coals and other coarse solid fuels.
- 4.
Fuel is also atomized in spark ignition engines by using a carburetor. However, the atomization is achieved by air turbulence near the point of fuel entrainment, rather than by a large pressure difference across a nozzle.
- 5.
The science of psychrometry deals with the properties of air–water mixtures.
- 6.
There are many situations, other than combustion involving heterogeneous reactions. For example, in catalysis, one species from the gaseous phase is preferentially adsorbed at the surface via a heterogeneous reaction. Surfaces with catalytic agents are often used in pollution abatement and other applications.
- 7.
In psychrometry, it is customary to define the specific humidity W \(=\) kg of water vapor/kg of dry air \(= m_{v} / m_{a}\). However, using the ideal gas relation, \(p_{v}\, V = m_{v}\, R_{v}\, T\) and \(p_{a}\, V = m_{a}\,R_{a}\, T\). Therefore, taking the ratio
$$W = \frac{m_{v}}{m_{a}} = \frac{R_{a}}{R_{v}} \times \frac{p_{v}}{p_{a}} = \frac{M_{v}}{M_{a}} \times \frac{p_{v}}{p_{a}} \simeq 0.622 \times \frac{p_{v}}{p_{tot} - p_{v}}\,,$$and, the mass fraction can be deduced as
$$\omega _{v} = \frac{\text{ kg } \text{ of } \text{ water } \text{ vapor }}{\text{ kg } \text{ of } \text{ air } + \text{ water } \text{ vapor }} = \frac{W}{1 + W}\,. $$Thus, in the w state, \(W_{w}\) and \(\omega _{v, w}\) can be calculated using \(p_{v} = p_{sat}\,(T_{w})\). Also, the \(\infty \)-state is prescribed as \(p_{v, \infty } = \Phi \times p_{sat}(T_{\infty })\). Therefore, \(W_{\infty }\) and \(\omega _{v, \infty }\) can be calculated knowing the relative humidity \(\Phi \).
For computer applications, the saturation conditions ( corresponding to \(\Phi = 1\)) for the range \(-\, 20< T_{w}\, (C) < 100\) can be correlated as
$$\begin{aligned} \omega _{v, w}\simeq & {} 3.416 \times 10^{-3} + (2.7308 \times 10^{-4})\, T_{w} + (1.372 \times 10^{-5})\, T^{2}_{w}\\+ & {} (8.2516 \times 10^{-8})\, T^{3}_{w} - (6.9092 \times 10^{-9})\, T^{4}_{w} + (3.5313 \times 10^{-10})\, T^{5}_{w} \\- & {} (3.7037 \times 10^{-12})\, T^{6}_{w} + (6.1923 \times 10^{-15})\, T^{7}_{w}\\+ & {} (9.9349 \times 10^{-17})\, T^{8}_{w} \end{aligned}$$ - 8.
In cooling towers and spray dryers, atomized liquid droplets fall under the action of gravity. The free-fall or terminal velocity of the droplet is given by
$$\begin{aligned} u_{p, t} = \sqrt{\frac{4\,g\, D_{p}}{3\, C_{d}}\,\left( \frac{\rho _{p}}{\rho _{air}} - 1\right) }\,\, \qquad \qquad \qquad {(10.16)} \end{aligned}$$where g is the acceleration due to gravity and \(C_{d}\) is the drag coefficient. The latter is correlated with Reynolds number Re as
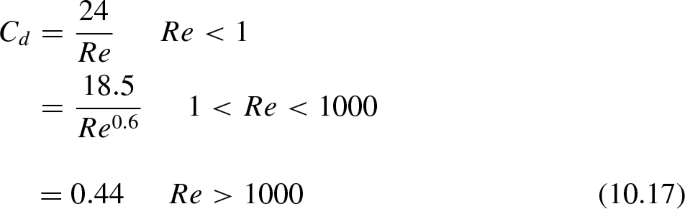
- 9.
An approximate answer can be developed if \(r_{w}\) in the denominator of Eq. 10.20 is replaced by the mean radius \(0.5 \times r_{w, i}\). Then, analytical integration gives
\(t_{eva} = \rho _{l}\, r^{2}_{w, i} \times \left[ 2\,\Gamma _{h}\,\text{ ln }\,(1 + B)\,(1 + C\,\sqrt{0.5 \times r_{w, i}}) \right] ^{-1} = 2.005\,\text{ s. } \)
- 10.
Boiling points of fuels are given in Appendix C.
- 11.
This fact is used in transpiration cooling of solid surfaces, in which a coolant gas is injected through the surface.
- 12.
Equation 10.54 is valid as long as pressures are low. For hydrocarbon fuels, critical pressures vary from 10 to 45 atm. If the pressure is high, \(h_{fg} \rightarrow 0\) and \(B \rightarrow \infty \). As such, the quasi-steady assumption must break down and one must adopt unsteady analysis, in which \(Q_{l}\) is evaluated from Eq. 10.34. High pressures are encountered in IC engines and in modern gas turbine plants.
- 13.
For more than 200 species of biomass, Channiwala [23] has correlated the HHV (MJ/kg) as
$$\begin{aligned} HHV= & {} 0.3491 \times \% \mathrm{C} + 1.1783 \times \% \mathrm{H} - 0.1034 \times \% \mathrm{O} \\- & {} 0.0211 \times \% \mathrm{A} + 0.1005 \times \% \mathrm{S} - 0.0151 \times \% \mathrm{N}\,, \end{aligned}$$where A is Ash. All percentages are on a mass basis.
- 14.
During drying, the temperature difference (\(T_{F} - T_{p}\)) is large. Hence, the variation of \(T_{p}\) with time is ignored.
- 15.
For example, water vapour can heterogeneously react with surface char to produce CO and H\(_2\). The latter can further react with the surface to produce CH\(_4\). As such, two additional reactions are
$$R5:\,\mathrm{C}^{*} + \mathrm{H}_{2}\mathrm{O} \rightarrow \mathrm{CO} + \mathrm{H}_{2} \text{ and } R6:\,\mathrm{C}^{*} + \mathrm{H}_{2} \rightarrow \mathrm{CH}_{4}\,,$$with \(k_{5} \simeq 2\, k_{3}\) and \(k_{6} = 0.035\,\exp {(-\, 17900 / T_{w})}\).
- 16.
If char is highly reactive \( \omega _{\mathrm{O}_{2}, w} \rightarrow 0\) and \(B_{m} \rightarrow \omega _{\mathrm{O}_{2}, \infty } / r_{st1} = 0.232 / 2.667 = 0.087\) (a small number). Therefore, ln\(\,(1 + B_{m}) \rightarrow B_{m}\) and
$$\begin{aligned} \dot{m}_{w} = 4\,\pi \,\Gamma _{m}\, r_{w}\,\left( \frac{\omega _{\mathrm{O}_{2}, \infty }}{r_{st1}}\right) \,. \qquad \qquad \qquad {(10.86)} \end{aligned}$$This expression was derived by Nusselt in 1924 [113].
- 17.
When \(T_{w}\) is small, \(k_{1}\) is also small.
- 18.
This theory for estimation of burn time assumes that the particle, when fully burned, vanishes in size. In reality, ash remains in the particle and, therefore, the fully burned particle does have a finite radius. Also, during burning, particle swelling may take place.
- 19.
This neglect stems from the high value of \(T_{w}\) which renders \(\omega _{\mathrm{O}_{2}, w} \simeq 0\) and hence, \(\dot{m}_{\mathrm{O}_{2}, w} = \dot{m}_{w1} = \dot{m}_{w2} \simeq 0\).
- 20.
Also, \( \dot{m}_\mathrm{CO} = (1 + r_{st_{3}})\,\dot{m}_{w}\).
- 21.
- 22.
Without iterations, Eq. 10.111 gives \(\omega _{\mathrm{CO}_{2}, w} = 0.177\).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Date, A.W. (2020). Combustion of Particles and Droplets. In: Analytic Combustion. Springer, Singapore. https://doi.org/10.1007/978-981-15-1853-9_10
Download citation
DOI: https://doi.org/10.1007/978-981-15-1853-9_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1852-2
Online ISBN: 978-981-15-1853-9
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)