Abstract
Transmissible gastroenteritis virus (TGEV), porcine epidemic diarrhoea virus (PEDV), and porcine deltacoronavirus (PDCoV) are enteropathogenic coronaviruses (CoVs) of swine. TGEV appearance in 1946 preceded identification of PEDV (1971) and PDCoV (2009) that are considered as emerging CoVs. A spike deletion mutant of TGEV associated with respiratory tract infection in piglets appeared in 1984 in pigs in Belgium and was designated porcine respiratory coronavirus (PRCV). PRCV is considered non-pathogenic because the infection is very mild or subclinical. Since PRCV emergence and rapid spread, most pigs have become immune to both PRCV and TGEV, which has significantly reduced the clinical and economic importance of TGEV. In contrast, PDCoV and PEDV are currently expanding their geographic distribution, and there are reports on the circulation of TGEV-PEDV recombinants that cause a disease clinically indistinguishable from that associated with the parent viruses. TGEV, PEDV and PDCoV cause acute gastroenteritis in pigs (most severe in neonatal piglets) and matches in their clinical signs and pathogenesis. Necrosis of the infected intestinal epithelial cells causes villous atrophy and malabsorptive diarrhoea. Profuse diarrhoea frequently combined with vomiting results in dehydration, which can lead to the death of piglets. Strong immune responses following natural infection protect against subsequent homologous challenge; however, these viruses display no cross-protection. Adoption of advance biosecurity measures and effective vaccines control and prevent the occurrence of diseases due to these porcine-associated CoVs. Recombination and reversion to virulence are the risks associated with generally highly effective attenuated vaccines necessitating further research on alternative vaccines to ensure their safe application in the field.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Porcine coronaviruses
- Transmissible gastroenteritis virus
- Porcine epidemic diarrhoea virus
- Porcine deltacoronavirus
- Enteropathogens
- Porcine respiratory coronavirus
- Pathology
- Detection
- Vaccine
4.1 Prologue
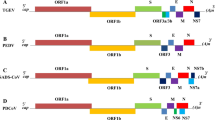
All known porcine coronaviruses (CoVs) belong to the genera Alphacoronavirus, Betacoronavirus and Deltacoronavirus of the subfamily Coronavirinae, in the family Coronaviridae, of the order Nidovirales (de Groot et al. 2008) [https://data.ictvonline.org/taxonomy-search.asp?msl_id=30 (Fig. 4.1)]. Affections of gastrointestinal, respiratory, peripheral and central nervous systems are usually visualised. Five swine CoVs are recognised: (1) the transmissible gastroenteritis virus (TGEV), first defined in 1946; (2) the porcine respiratory coronavirus (PRCV), a mutant of TGEV, isolated in 1984; (3) the porcine epidemic diarrhoea virus (PEDV), isolated in 1977; (4) the PHEV (porcine haemagglutinating encephalomyelitis virus) isolated in 1962; and (5) the PDCoV (porcine deltacoronavirus) described in 2012. The first two, TGEV and PRCV, belong to the Alphacoronavirus 1 species together with closely associated CoVs of cats and dogs, and PEDV and human CoVs (229E and NL63) form distinct species in the Alphacoronavirus genus. PHEV and PDCoV belong to the Beta- (Betacoronavirus 1 species) and Deltacoronavirus genera, respectively. PDCoV is closely related to the deltacoronaviruses from Asian leopard cats and Chinese ferret badgers (Ma et al. 2005). While PRCV induces primarily subclinical infections in pigs, enteropathogenic swine alphacoronaviruses (TGEV, PEDV, SeCoV, porcine enteric alphacoronavirus) and PDCoV are allied with a severe enteric disease of variable severity depending on the animal age and immune status. One serotype is recognised for each swine CoV species.
TGEV and PEDV have been reportedly co-circulating in Eurasia and the USA. Recently in Europe, a pathogenic recombinant TGEV/PEDV variant (swine enteric coronavirus, SeCoV) was recognised and described (Akimkin et al. 2016; Belsham et al. 2016; Boniotti et al. 2016). SeCoV that contains PEDV S protein on a TGEV backbone apparently leads to disease clinically indistinct from the TGEV- and PEDV-produced ones (Table 4.1). Additionally, a novel bat-HKU2-like porcine coronavirus [porcine enteric alphacoronavirus (PEAV), GDS04 strain] associated with severe diarrheal disease in suckling piglets was identified in Southern China in 2017 (Gong et al. 2017) (Table 4.1). However, its prevalence and adaptation status to the swine host are unknown.
Currently, PHEV, the only porcine betacoronavirus, has a worldwide prevalence (Li et al. 2016). In neonatal piglets devoid of maternal antibodies (generally in those purchased from infection-free herds), PHEV causes either encephalomyelitis or a condition characterised by vomiting and wasting. Generally, maternal immunity protects piglets which have taken colostrum for up to 15 weeks, while in pigs older than 3–4 weeks and adult swine the infection is mostly subclinical. Therefore, it is seldom considered to be of economic importance. However, a recent report of an uncommon respiratory (influenza-like) presentation and increasing prevalence of PHEV in adult exhibition swine in the USA (Lorbach et al. 2017) may indicate a potential tropism shift that could lead to a substantial change in its epidemiology. To clear this complex epidemiological position, continuing monitoring and development of state-of-the-art rapid and reliable tools and techniques are needed to confirm and provide a clear differential diagnosis (Kim et al. 2001; Masuda et al. 2016).
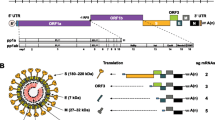
CoVs are enveloped and pleomorphic, 60–160 nm in diameter. Swine CoV shaves single-stranded, polyadenylated, large genomic RNA (25–30 kDa) of positive-sense polarity that is infectious. The genome profile, replication strategies as well as protein expression match to other human and animal CoVs (Enjuanes and Van der Zeijst 1995; Gonzalez et al. 2003; Laude et al. 1993). Most porcine CoVs have four basic structural proteins: a large surface glycoprotein (S, spike protein that forms a monolayer of club-shaped spikes defined as the corona); a small membrane protein (E); an integral membrane glycoprotein (M); and a nucleocapsid protein (N). However, PHEV contains a haemagglutinin-esterase (HE) protein that forms a second shorter layer of surface spikes (de Groot et al. 2008). TGEV, PEDV and PDCoV also transcribe 1–2 accessory proteins encoded by open reading frame (ORF)3 (TGEV and PEDV), ORF6 (PDCoV), and ORF7 (TGEV and PDCoV). The complete genome organisation is 5′UTR-ORF1ab, S, ORF3, E, M, ORF6, N and ORF7-3′UTR.
An overall nt and amino acid sequence similarity of 96–98% among TGEV and PRCV proposes that PRCV evolved from TGEV. Two characteristic features of the PRCV genome that may account for its altered tissue tropism include a large omission (621–681 nt) in the N-termini of the S gene resulting in a reduced S protein size and variable sequence changes in the ORF3 (Ballesteros et al. 1997; Sanchez et al. 1999).
While there is no evidence of the existence of different PEDV serotypes (Lin et al. 2015a), genetically, PEDV strains are classified into two groups: (1) classical (isolates from Eurasia that are genetically similar to the prototype CV777 strain) and (2) emerging PEDV strains (Lin et al. 2016; Vlasova et al. 2014). All classical PEDV strains contain inserts and omissions in the spike gene (S INDEL) that are not present in the majority of the highly virulent emerging PEDV strains (Vlasova et al. 2014). Thus, these highly virulent strains that originally emerged in China in 2010 and transmitted to the USA, Europe and other Asian parts are referred to as emerging non-S INDEL PEDV strains. Recombinants between these two major groups of PEDV contain a set of deletions-insertions in their spike gene identical to those of the classical strains. They are called S INDEL strains and circulate in Asia, Europe and the USA. Additionally, a few reports described other uncommon PEDV variants that bear large deletions (194–216 aa) in the N-terminal domain (NTD) of the S protein and designated as S1 NTD-del type of PEDV (Diep et al. 2017; Oka et al. 2014; Suzuki et al. 2015). Unlike the altered tissue tropism from enteric TGEV to respiratory PRCV, these (S INDEL and S1 NTF have been reported-del) PEDV strains have kept their enteric predilection, but with lower virulence (Suzuki et al. 2016; Hou et al. 2017).
Swine enteric CoVs (TGEV, PEDV and PDCoV) are highly contagious and are associated with severe disease forms such as diarrhoea and vomition, and increased mortality in young ones (often 100%). They can cause sporadic outbreaks (endemic) or large-scale epidemics in swine-producing countries (Fig. 4.2). No specific treatments are available for any of the swine enteric CoVs that so far have resisted eradication efforts in different geographic regions. In this chapter, we have reviewed the diseases due to CoVs that continue evolving in domestic and wild swine, as well as another possible reservoir (avian or bat species) or secondary hosts including carnivores, or via the interspecies spread, recombination and generation of deletion escape variants. We also review PRCV that has lost its enteric tropism but is capable of inducing protective immune responses against TGEV that altered its global epidemiology.
4.2 Pathogenesis and Clinical Signs
4.2.1 TGEV
Extensive necrosis of mature enterocytes of jejunum and ileum within 24 h after infection results in reduced enzymatic activity (alkaline phosphatase, lactase, etc.), disrupted digestion, and cellular electrolyte (including sodium) balance. These changes primarily lead to the deposition of fluid in the intestinal lumen, acute malabsorptive diarrhoea (Moon 1978). The loss of extravascular protein and copious dehydration in piglets can be fatal (Butler et al. 1974). The latter can also lead to metabolic acidosis and hyperkalaemia, causing abnormal cardiac function.
TGE gross lesions are limited to the gastrointestinal tract. The distension of the stomach and the small intestine are seen to be filled up with curdled milk and sometimes petechial haemorrhages are visualised (Hooper and Haelterman 1966a). The small intestinal wall is thin and transparent. The villous atrophy in the jejunum and lesser in the ileum regions are the major TGE lesions and are more pronounced in neonatal pigs than in ≥3-week-old piglets (Moon 1978; Hooper and Haelterman 1966b). The increased severity of TGEV infection results in higher mortality (often 100%) in piglets less than 2 weeks of age that decreases in older pigs (Table 4.1). Although swine of any age is susceptible to TGEV, the mortality in TGEV seropositive groups and swine more than 5 weeks of age is usually low. Mechanisms that represent age-dependent susceptibility to clinical ailment comprise the slower substitution of tainted villous epithelial cells with newly differentiated enterocytes migrating from crypts in newborn pigs (Moon 1978). These lesions are similar to PEDV/PDCoV (Debouck et al. 1981; Jung et al. 2015a) lesions, but more severe than those caused by rotavirus (RV) (Bohl et al. 1978). Pathologic observations and degree of villous atrophy are exceptionally variable in pigs from endemic herds (Pritchard 1987).
Lungs (alveolar macrophages) and mammary gland tissues are recognised extra-intestinal sites for TGEV replication (Kemeny et al. 1975). Hitherto report shows pneumonia due to oronasal infection of pigs with TGEV (Underdahl et al. 1975), and the clinical significance of mammary gland infection is imprecise. However, agalactia is frequently observed in TGEV-affected sows, and TGEV spreads quickly among the population.
4.2.2 PRCV
PRCV replicates efficiently in porcine type 1 and 2 pneumocytes and is seen in epithelial cells of the nares, trachea, bronchi and bronchioles, and alveoli, and on occasion in alveolar macrophages (Atanasova et al. 2008; Pensaert et al. 1986; O’Toole et al. 1989). It can be noticed in blood and tracheobronchial lymph nodes. After experimental infection, nasal PRCV shedding usually lasts for 4–6 days. Pulmonary lesions and clinical signs subside consequently with an increase in the virus-neutralising (VN) antibody titres (Atanasova et al. 2008). Although PRCV is sometimes found in enterocytes, it does not spread efficiently to adjacent epithelial cells (Cox et al. 1990), and faecal shedding is low or undetectable.
PRCV predominantly causes upper and lower respiratory tract disease. The lesions appear to include lung and bronchointerstitial pneumonia, with regular peribronchiolar and perivascular lymphohistiocytic handcuffing (Atanasova et al. 2008; Cox et al. 1990; Halbur et al. 1993; Jung et al. 2007). The PRCV-induced bronchointerstitial pneumonia results in (1) thickened alveolar septa due to macrophage and lymphocyte infiltration, (2) hypertrophy and hyperplasia of type 2 pneumocytes, (3) aggregation of cell debris and inflammatory leukocytes in alveolar and bronchiolar lumina because of airway epithelial necrosis and (4) peribronchiolar or perivascular lymphohistiocytic inflammation.
4.2.3 PEDV
Clinical signs are evident between 22 and 36 h postinfection and match with the peak of viral replication (Table 4.1). The clinical presentation (watery malabsorptive diarrhoea, vomiting, depression and anorexia) and pathological lesions of PEDV are clinically indistinguishable from those of TGEV (Debouck et al. 1981; Coussement et al. 1982).
Morbidity is nearly 100% in piglets and variable in sows. Neonates below 1 week of age often die due to severe dehydration, and mortality touches 50–100%, whereas mortality is low in older pigs and they recover within a week. In sows, severity of diarrhoea is constant and frequently shows only depression and anorexia. Similarly, fattening pigs may develop watery faeces and can become anorexic and depressed within a week. As with TGEV, slower enterocyte turnover and immature innate immune system may add to the more extreme clinical signs, higher mortality and slower recuperation in PEDV-tainted piglets in contrast to weaned pigs (Jung et al. 2015a; Moon et al. 1975; Annamalai et al. 2015).
Each outbreak generally lasts for ~3–4 weeks; however, it might be longer on large breeding farms with multiple, isolated units and variable levels of lactogenic immunity in gilts/sows. PEDV-exposed pregnant sows can provide sufficient lactogenic immunity to protect their piglets, and PED outbreaks stop. After the passage of acute outbreak, diarrhoea may persist and is recurrent in weaned pigs, resembling endemic TGE form (Martelli et al. 2008).
The severity of lesions and the virus replication levels in naturally and experimentally infected suckling piglets vary for classical PEDV, emerging non-S INDEL and S INDEL PEDV strains (Jung et al. 2015a; Coussement et al. 1982; Kim and Chae 2003; Pospischil et al. 1981; Sueyoshi et al. 1995; Lin et al. 2015b; Madson et al. 2014). Lesions remain localised to the small intestine that is swollen and filled up with watery, yellowish liquid. Microscopic examination shows syncytia, vacuolation and shedding of small intestinal enterocytes primarily on the proximal villi. Similar to TGEV, PEDV infection results in degeneration of enterocytes that reduces the villous height:crypt depth (VH: CD) ratios and the enzymatic activity. Although PEDV antigens were detected in colonic epithelial cells, no associated histopathologic changes have been observed (Debouck et al. 1981).
Viral RNA has been confirmed in the serum, and different tissues (including lung, spleen, liver and muscle) of pigs euthanised during PEDV infection (Suzuki et al. 2016; Jung et al. 2014, 2015a; Lohse et al. 2017; Chen et al. 2016a; Park and Shin 2014) with high RNA titres in the serum of 7–8 log10 GE/mL coinciding with peak RNA titres in faeces (11–12 log10 GE/mL) (Jung et al. 2015a). Additionally, PEDV RNA is identified in 40.8% (20/49) of sow milk samples during the epidemics caused by emerging PEDV strains (Sun et al. 2012).
4.2.4 PDCoV
The clinical signs are observed within 1–3 days after PDCoV infection in suckling and older pigs. Although clinical symptoms are similar (Table 4.1), they are less pronounced compared to PEDV and TGEV infections (Chen et al. 2015; Hu et al. 2016; Jung et al. 2015b; Ma et al. 2015). They include acute, watery diarrhoea due to malabsorption induced by the massive loss of absorptive enterocytes. Additional signs may include vomiting, dehydration, weight loss, lethargy and death. Vacuolation of the infected colonic epithelial cells may inhibit water and electrolyte reabsorption. The seronegative pigs are susceptible to PDCoV infection at any age, with high morbidity that can reach 100% in piglets. Evaluation of filed cases in the USA, China and Thailand in 2014 shows that PDCoV infection is associated with up to 40–80% mortality among suckling pigs (Anon 2014). The infection on breeding establishments remains self-limiting and stops when pregnant sows develop lactogenic immunity adequate to secure their offspring.
Gross lesions include thinned and transparent intestinal walls (jejunum to the colon) with a collection of a lot of yellow liquid with gas. Often stomach is found bloated with curdled milk.
PDCoV replicates in the epithelial cell of the large and small intestine. Lesions look like those seen in TGEV and PEDV infections but are mild (Chen et al. 2015; Hu et al. 2016; Jung et al. 2015b; Ma et al. 2015). Histological findings are intense, multifocal to diffuse, mild to extreme atrophic enteritis of jejunum and ileum, at some point joined by mild vacuolation of caecal and colonic epithelial cells (Jung et al. 2015b). Amid acute infection, PDCoV antigens are available in the villous epithelium of the mid-jejunum to the ileum and a lesser degree, in the duodenum, and caecum/colon (Jung et al. 2016a). PDCoV antigens may also be noticed in immune cells of the intestinal lamina propria, Peyer’s patches and mesenteric lymph nodes (Hu et al. 2016). Inflammatory cell (macrophage, lymphocyte and neutrophil) infiltration can be observed in the lamina propria. Acute necrosis of PDCoV-infected enterocytes (Jung et al. 2016a) results in marked villous atrophy in jejunum and ileum, but not duodenum or large intestine, which coincides with fewer PDCoV antigen-positive duodenal, caecal or colonic epithelial cells (Chen et al. 2015; Jung et al. 2015b). Acute-phase viremia with low PDCoV RNA titres in serum is observed (Chen et al. 2015; Hu et al. 2016). After recovery of pigs from clinical disease, huge amount of PDCoV antigens are found in the gut lymphatic tissues (Hu et al. 2016). Additionally, low or moderate quantities of PDCoV RNA, but not antigens, are detected in multiple organs, feasibly as of viremia (Chen et al. 2015; Ma et al. 2015; Jung et al. 2016b). Decreased levels of PDCoV shedding (compared with PEDV and TGEV) in the faeces may be indicative of its incomplete adaptation to pigs and can contribute to its slower spread among swine herds and the lower mortality of nursing pigs (Jung et al. 2015b).
4.3 Incidence and Prevalence of the Disease
4.3.1 TGEV
TGEV was first detected in the USA in 1946 from outbreaks of acute diarrhoea with high mortality in piglets (Doyle and Hutchings 1946). Since then the disease has been reported in several pig-rearing countries practicing intensive pig farming system, including Europe, Asia (Japan, Korea, Malaysia and Taiwan), the Americas (North, Central and South) and Africa (Zaïre, Ghana). Despite the widespread application of vaccines, TGEV infections were a prime reason for enteric disease and mortality in piglets in the USA and globally in the 1960s–1980s. The presence and extensive prevalence of PRCV, a deletion mutant of TGEV, narrowed the clinical impact of TGE (Laude et al. 1993; Pensaert et al. 1986, 1993; Brown and Cartwright 1986; Pensaert 1989; Yaeger et al. 2002). Currently, sporadic outbreaks of profuse diarrhoea in piglets due to TGEV in TGEV/PRCV seronegative herds are yet to be confirmed in North America, Europe and Asia. However, careful differentiation between TGEV and emerging TGEV/ PEDV recombinants may be needed.
Two epidemiologic forms of TGE are apparent: epidemic and endemic. Epidemic TGE noticed transcendently in seronegative flocks. After entry, the illness transmits quickly to swine of any age, particularly in winters, with inappetence, vomition or diarrhoea in affected animals. Suckling pigs exhibit prominent clinical signs and get quickly dehydrated. Lactating sows usually show anorexia and agalactia, with reduced milk production, which further adds to piglet mortality.
Endemic TGE indicates the persistence of the virus and disease in a group perpetuated by the continuous influx of susceptible swine. It is a classic sequel to a primary outbreak and occurs in seropositive animals that regularly have farrowing (Stepanek et al. 1979), additions of the herd or mixing of susceptible pig population. In endemic groups, TGEV spreads slowly among grown-up pigs (Pritchard 1987). Sows are most of the time resistant and asymptomatic and will transfer a variable level of passive lactogenic immunity to their offspring. In these groups, mild TGEV diarrhoea is seen with mortality under 10–20% in pigs from ~6 days of age until ~2 weeks post-weaning.
4.3.2 PRCV
PRCV infects the respiratory tract with limited or no shedding in faeces (Pensaert 1989). The first isolation of PRCV was from Belgium in 1984 (Pensaert et al. 1986) and 1989. PRCV was detected in the USA in the herds without a prior history of TGEV infection or vaccination (Hill et al. 1990; Wesley et al. 1990). Antibodies produced in PRCV-infected pigs neutralise the TGEV.
Since the first report, the virus has been introduced rapidly in Europe (Laude et al. 1993; Brown and Cartwright 1986; Have 1990; van Nieuwstadt et al. 1989) and attained endemic status worldwide, including entering TGEV-free countries (Laude et al. 1993; Pensaert 1989; Pensaert et al. 1993). A serological survey from the USA in 1995 demonstrated that clinically healthy pigs from different herds were found to be seropositive for PRCV (Wesley et al. 1997) in Iowa State.
4.3.3 PEDV
The classical PEDV strains were the cause of several epidemics with heavy mortality in Europe from 1971 until the late 1980s. However, after 2000, reports are very rare. In Italy, an epidemic involving 63 herds occurred in 2005 and 2006 where pigs of all ages were found affected, but mortality was mainly limited to suckling piglets (Martelli et al. 2008). Because of the low clinical importance of the disease, no surveillance studies were conducted until the emergence of new PEDV variants in Europe in 2014. Note that the historical prevalence of classical PEDV in the European swine population is unknown. An emerging non-S INDEL strain led to an outbreak in Ukraine in 2014; while outbreaks in European countries (Germany, Belgium, France, the Netherlands and Slovenia) were confirmed as of S INDEL strains (Lin et al. 2016).
Infections associated with classical PEDV strains were originally reported in China in the late 1970s. Since then, PED has spread among swine farms and became leading cause of viral diarrhoea, despite the use of vaccines (targeting the prototype PEDV strain CV777) (Wang et al. 2016a; Xuan et al. 1984). In Japan PEDV was first detected in 1982 (Takahashi et al. 1983) and outbreaks continued during the 1990s with mortality between 30% and 100% in suckling pigs (Sueyoshi et al. 1995; Kuwahara et al. 1988). PEDV was first recognised in Korea in 1993 (Kweon et al. 1993) and became prevalent accounting for more than 50% of the enteric viral infections diagnosed in suckling piglets in the 1990s (Chae et al. 2000; Hwang et al. 1994). In India, 21.2% of 528 serum samples from pigs (2–6 months old) were confirmed positive for PEDV antibodies (Barman et al. 2003). In Thailand, between 2007 and 2008, PED occurred in eight provinces (Puranaveja et al. 2009) affecting all age-group pigs but mortality reached 100% in newborn piglets.
In October 2010, a massive series of PEDV outbreaks occurred in China, resulting in tremendous economic losses (Sun et al. 2012, 2016). These outbreaks were caused by the emerging extremely virulent non-S INDEL strains, and the mortality in neonatal piglets reached 50–100% (Wang et al. 2016b). Subsequently, emerging S INDEL strains were also identified in China (Wang et al. 2016b), demonstrating that classical and emerging (non-S INDEL and S-INDEL PEDV) strains are co-circulating in China.
In 2013, the emerging PEDV strains started spreading to other countries outside China. At the same period, outbreak due to highly virulent non-S INDEL PEDV strains started in US swine farms (Stevenson et al. 2013), which was followed by outbreaks associated with milder S-INDEL PEDV strains in January 2014 (Wang et al. 2014a). However, S INDEL strains were retrospectively found in pig samples collected as early as June 2013 (Vlasova et al. 2014). In 2013–2014, PEDV led to the loss of nearly 10% of the US swine stock (7 million pigs) and the associated profits. By 2018, PEDV had reportedly spread to 39 US states and Puerto Rico (www.aphis.usda.gov/animal-health/secd). In the USA, the last big outbreak of PEDV occurred in the spring of 2014, and the emerging non-S INDEL PEDV strains have reportedly spread to the neighbouring countries, including Canada and Mexico (Lin et al. 2016).
In 2013, the emerging non-S INDEL PEDV strains were identified in Japan (Masuda et al. 2015) and then reportedly spread to South Korea (Kim et al. 2015), Vietnam (Vui et al. 2014), Thailand (Cheun-Arom et al. 2015), Taiwan (Lin et al. 2014) and the Philippines (Kim et al. 2016). The S-INDEL PEDV strains were detected in Japanese (Suzuki et al. 2015) and Korean (Lee et al. 2014) farms in 2013 and 2014, respectively. To date, other PEDV variants that carry a large deletion in the NTD of the S protein were detected exclusively in pigs in Japan. Besides the avirulent TTR-2 strain (Suzuki et al. 2016), 15 novel field PEDV mutants with large deletions in the NTD of S gene ranging from 582 to 648 nt were detected from diarrhoeic pig faecal or intestinal samples collected between December 2013 and June 2015 (Diep et al. 2017). Interestingly, all of these samples contained at least two PEDV strains with distinct large genomic deletions, and the majority of these PEDV strains were confirmed to contain an intact S gene. These variants with large deletions in the S gene were found in both primary and recurring PED outbreaks. In summary, classical, emerging non-S INDEL and S-INDEL PEDV strains exist in Asia and Europe, but only emerging non-S INDEL and S-INDEL PEDV strains are currently confirmed to circulate in the Americas. No PEDV infections have been reported in the African and Australian continents so far.
4.3.4 PDCoV
The initial surveys in China and Hong Kong during 2007–2011 identified DCoVs in pigs and wild birds (Woo et al. 2012). Nonetheless, the DCoV presence was previously confirmed at Chinese live-animal markets in 2005–2006 in small mammals, including Chinese ferret badgers and Asian leopard cats (Dong et al. 2007). Because their helicase and S genes are closely related with PDCoV, interspecies transmission of DCoVs among Asian carnivores, porcine and avian species is suggested. The origin of PDCoV remains unclear, but considering its recent emergence, its adaptation to pigs may be incomplete.
In early 2014, the first outbreak of PDCoV-associated diarrhoea was documented in swine, in Ohio. Among intestinal or faecal samples obtained from diarrhoeic pigs from 5 Ohio farms, 92.9% were found to be positive for PDCoV by RT-PCR (Wang et al. 2014b). The PDCoV sequences shared high nucleotide similarity with the two Chinese prototype PDCoV strains, HKU15-44 and HKU15-155, identified in 2012. Other two genetically similar PDCoV strains, USA/IA/2014/8734 and SDCV/USA/Illinois121/2014, were detected in US states during the same time period (Li et al. 2014; Marthaler et al. 2014a). After this PDCoV has been confirmed in 19 US states (www.aphis.usda.gov/animal-health/secd) but is still less widespread than PEDV. PDCoV origin in US swine is unknown; however, serologic evidence suggests that it was circulating in US swine before its recognition in 2014 (Sinha et al. 2015; Thachil et al. 2015).
PDCoV has also been documented in Canada (March 2014), Korea (April 2014), mainland China (2015), Thailand (2015) and Vietnam and Lao PDR. A Korean study described that of 691 faecal samples from 59 farms collected from diarrhoeic pigs from 2014 to 2015, only 2 samples originating from a single farm were PDCoV RNA positive (Lee et al. 2016a). The two Korean PDCoV strains, SL2 and SL5, were genetically closely related to the US PDCoV strains but differed from the older Korean strain KNU14-04. Following the first PDCoV identification in Hong Kong in 2012 (Woo et al. 2012), it was confirmed in diarrhoeic pigs in mainland China (Dong et al. 2015; Song et al. 2015). PDCoV high prevalence (>30%) and frequent co-infections with PEDV (51%) were reported. All Chinese PDCoV strains shared high nt identities (≥98.9%) with the global PDCoV strains. The Thai PDCoV strains shared the highest nt identities (≥98.4%) with the Chinese strain CHN-AH-2004 (Janetanakit et al. 2016), forming, however, a cluster separate from Chinese and US strains (Zhang 2016). Strains genetically close to the Thai PDCoV lineage were found in Lao PDR, whereas strains of the US PDCoV lineage were detected in Vietnam (Saeng-Chuto et al. 2017).
4.4 Immunobiology
4.4.1 TGEV/PRCV
Infection of adult swine results in noticeable serum antibodies that could persist for 6 months to several years (Stepanek et al. 1979). Serum antibody presence confirms serological evidence for TGEV or PRCV infection; their association with protection against TGEV is not established. Swine that recover from TGEV infection show protection from subsequent short-term challenge, as of intestinal mucosal immunity (Brim et al. 1995; Saif et al. 1994; VanCott et al. 1993, 1994).
Protective immunity depends on the secretory IgA (sIgA) antibodies produced by intestinal plasma cells (Saif et al. 1994; VanCott et al. 1993, 1994). Intestinal and serum IgA TGEV antibodies and antibody-secreting cells (ASCs) have been detected in pigs after oral, but not after parenteral, administration with TGEV (Saif et al. 1994; VanCott et al. 1993, 1994; Kodama et al. 1980). Presence of IgA antibody (likely of intestinal origin) in the serum is considered to be an indicator of active immunity to TGE (Kodama et al. 1980; Saif 1999). Besides local antibody responses, cell-mediated immunity (CMI) is also important in protection against TGEV infections (Brim et al. 1995; Frederick et al. 1976; Shimizu and Shimizu 1979). Retinoic acid may enhance CMI after immunisation of piglets with an inactivated TGEV vaccine by increasing the trafficking of CD8+ T cells to lymph nodes and small intestine (Chen et al. 2016b). It was conceived that a low natural killer (NK) cell activity might associate with the higher susceptibility of piglets and parturient sows to TGEV infection (Cepica and Derbyshire 1984). A virulent (SHXB) however not attenuated (STC3) TGEV strain impeded the capacity of porcine intestinal DCs or monocyte-derived DCs to sample antigen, migrate and incite T-cell expansion in vivo and in vitro (Zhao et al. 2014), suggestive of the immune-suppressive potential of TGEV.
PRCV-inoculated pigs developed potent systemic and bronchus-associated but not gut-associated antibody, ASC and T-cell responses (VanCott et al. 1993; Brim et al. 1994). Other than quantitative differences in the count of IgA antibodies in the sow milk induced by TGEV or PRCV exposure, putative divergences in virus epitopes observed by the milk IgA antibodies were recommended (De Diego et al. 1992).
Subsequently to extensive decrease in epidemic outbreaks of TGE in Europe following the widespread distribution of PRCV, the research suggested that PRCV infections induce partial immunity against TGEV, as proven by a reduced length and duration of virus shedding and diarrhoea in most of the pigs examined (Brim et al. 1995; VanCott et al. 1994; Cox et al. 1993; Wesley and Woods 1996). This partial immunity is associated with rapid increases in TGEV-VN antibodies (Cox et al. 1993; Wesley and Woods 1996) and numbers of IgG and IgA ASCs in the intestines (Saif et al. 1994; VanCott et al. 1994). Movement of PRCV IgG and IgA ASCs from the bronchus-associated lymphoid tissues (BALT) to the gut of the PRCV-exposed pigs after TGEV challenge may clarify the quick anamnestic response and induces the partial protection (VanCott et al. 1994). However, neonatal pigs needed a week after PRCV encounter to develop partial immunity to subsequent TGEV challenge (Wesley and Woods 1996).
Circulating passive antibodies (primarily IgG), acquired with colostrum, protect the neonate against systemic, however not the intestinal, infection (Hooper and Haelterman 1966a; Saif and Sestak 2006). Between the first 7 days of lactation, IgA prevails in milk and IgG reduces. Researchers have reviewed the mechanisms of passive immunity to TGEV infection (Saif and Sestak 2006; Chattha et al. 2015; Saif and Jackwood 1990; Saif and Bohl 1979). Swine recovered from TGE spread passive immunity to their suckling pigs with colostrum or milk (lactogenic immunity) containing TGEV virus-neutralising antibodies (Hooper and Haelterman 1966a) that nullify the ingested TGEV in the intestinal lumen. This is accomplished naturally when piglets feed on immune mother regularly or experimentally by constant feeding of antiserum.
IgA TGEV antibodies in milk are stable in the gut and give the best protection, but IgG antibodies are likewise protective if high titres are restored in milk after parenteral or systemic vaccination (Bohl and Saif 1975) or by ingestion of colostral IgG antibodies (Stone et al. 1977). Following TGEV infection and antigenic stimulation in the gut, IgA immunocytes move to the mammary gland where they confine and produce IgA antibodies into colostrum and milk that play a major role in passive protection of suckling pigs (Saif and Sestak 2006; Saif and Jackwood 1990; Saif and Bohl 1979; Bohl and Saif 1975). This “gut-mammary” immunologic axis was first proposed concerning TGEV infections in swine (Bohl et al. 1972; Saif et al. 1972), providing the first concept for a common mucosal immune system.
4.4.2 PEDV
Even though all age pigs are susceptible to PED, highest mortality is seen in 1-week piglets and younger, and their survival is dependent on the transfer of maternal antibodies, especially VN and sIgA (via colostrum and milk from immunised or previously exposed sows). The components of lactogenic security depicted for TGEV infection apply to PEDV also (described previously in TGEV section) (Chattha et al. 2015; Langel et al. 2016). Pigs lose lactogenic protection at weaning becoming susceptible to PEDV infection. Humoral immune response to PEDV infection is quite the same as seen for TGEV (described in TGEV section) (Saif and Sestak 2006; Chattha et al. 2015). VN antibodies appear in the serum but do not play any significant role in protection against the clinical disease since protection basically relies on the presence of slgA antibodies in the intestinal mucosa (Chattha et al. 2015; Langel et al. 2016). Immunity may not persist long, yet a fast anamnestic response upon re-exposure may decrease the severity of reoccurring disease or even prevent it.
At least 11 proteins [ORF1ab-encoded NS proteins (nsp1, nsp3, nsp5, nsp7, nsp14–16), structural proteins (E, M, N) and accessory protein ORF3s] have been recognised as IFN antagonists allowing PEDV to evade host interferon (IFN) responses (Ding et al. 2014; Wang et al. 2015; Zhang et al. 2016a). Decreased innate immune responses (specifically frequencies and function of NK cells) (Annamalai et al. 2015) likely contribute to the increased severity of PEDV infection in suckling vs. older (weaned, finisher, adult) pigs, as observed for TGEV infections (Derbyshire et al. 1969).
4.4.3 PDCoV
The immune response to PDCoV infection in pigs is still unclear but presumed to be similar to that mentioned for TGEV and PEDV. Gnotobiotic pigs orally inoculated with the original or tissue-culture-developed PDCoV strain (OH-FD22) exhibited serum IgG, IgA and VN antibodies by 14 dpi that shows a peak at 24 dpi when the pigs had recouped from the clinical form and faecal virus shedding (Hu et al. 2016). Similar to TGEV and PEDV, the supply of maternal antibodies with colostrum and milk from immune sows, especially IgA and VN antibodies, should neutralise PDCoV in the gut protecting young piglets (Bohl et al. 1972; Saif et al. 1972).
4.5 Diagnosis
4.5.1 TGEV/PRCV
Since clinical signs and atrophic enteritis due to TGEV are also seen in other enteric infections (RV, PEDV, PDCoV and coccidia), lab-based diagnosis of TGE must be followed utilising the following tests: identification of viral antigen or nucleic acids in faeces, virus seclusion from samples or detection of TGEV antibodies.
Similar methods are used for the diagnosis of PRCV, but with an emphasis on respiratory specimens (nasal swabs or lung homogenates). Evaluation of clinical signs, histologic lesions and viral antigen distribution in tissues might yield a presumptive diagnosis.
Probing of TGEV antigen in small intestinal enterocytes by immunofluorescence (IF) (Pensaert et al. 1970) or immunohistochemical (IHC) (Shoup et al. 1996) techniques using monoclonal antibodies (MAb) against the highly conserved TGEV N protein may be conducted for formalin-fixed or frozen tissues harvested in the early stage of infection.
A mono- or polyclonal antibody-based enzyme-linked immunosorbent assay (ELISA) is utilised to recognise TGEV antigens in cell cultures, intestinal contents and faeces (Lanza et al. 1995; Sestak et al. 1996, 1999a; van Nieuwstadt et al. 1988) or PRCV antigen in nasal swabs or lung homogenates (Lanza et al. 1995); however, the sensitivity of available ELISA tests is generally lower than that of RT-PCR assays. RT-PCR or real-time qPCR is now more commonly used for detection of TGEV and distinguishing TGEV, PRCV, PDCoV and PEDV (Kim et al. 2000, 2001; Masuda et al. 2016; Costantini et al. 2004; Ogawa et al. 2009). PRCV/TGEV distinction is usually performed through PCR assay based on the S gene deletion region in PRCV strains. Multiplex RT-PCR and real-time qPCR have been used for the concurrent identification of major porcine viruses related to diarrhoea including RV, TGEV, PDCoV and PEDV (Masuda et al. 2016; Ogawa et al. 2009). Also, multiplex microarray hybridisation assay was used for quick differential identification of eight CoVs, including TGEV (Chen et al. 2005).
Transmission electron microscopy (TEM) is used to show TGEV in the faecal or intestinal contents of infected pigs. Further, immune EM (IEM) possesses many advantages over TEM and is more sensitive and better in differentiating TGEV from PEDV and PDCoV (Saif et al. 1977).
Primary and secondary pig kidney cells (Bohl and Kumagai 1965) or cell lines (Laude et al. 1981), porcine thyroid cells (Witte 1971) and McClurkin swine testicle (ST) cell line (McClurkin and Norman 1966) are advocated for TGEV isolation from infected pig faeces or intestinal contents. Typical cytopathic effects (CPE) might be negligible upon primary isolation of field strains and require additional blind passages. The CPE observed comprises swollen, round cells with a balloon-like appearance (Bohl and Kumagai 1965). For observing viral CPE or plaques, the addition of pancreatin or trypsin to the ST cell culture media as well as using older cells can improve detection of viral CPE and plaques (Bohl 1979).
ST cells and pig kidney cells are best for isolation of PRCV from a nasal swab or lung tissue homogenates. PRCV- and TGEV-induced CPE are comparable with syncytia usually seen that is also observed in PEDV grown in Vero cells (Hofmann and Wyler 1988; Ksiazek et al. 2003). Detection of virus in the cell culture is confirmed by VN, IF or IEM using specific TGEV antiserum or differential monoclonal antibodies (Garwes et al. 1988) and virus-specific RT-PCR (Enjuanes and Van der Zeijst 1995; Laude et al. 1993; Kim et al. 2000).
TGEV antibodies can be identified by several serological tests. However, TGEV serology is complex, as both TGEV and PRCV prompt VN antibodies that are quantitatively and qualitatively similar (Pensaert 1989). A blocking ELISA that uses MAbs to TGEV differentiates easily from PRCV (Garwes et al. 1988; Bernard et al. 1989; Callebaut et al. 1989). However, blocking ELISA works better on a group basis since certain pigs with low antibody titres to TGEV or PRCV may not show positivity (Callebaut et al. 1989; Sestak et al. 1999b; Simkins et al. 1993). Further, the accuracy of commercial ELISAs for distinguishing US strains of PRCV and TGEV is low (Sestak et al. 1999a).
4.5.2 PEDV
As for TGEV, diagnosis of PEDV must be based on clinical signs and lab identification of viral RNA, viral antigens or increased PEDV-species antibodies. For the location of PEDV RNA, the most broadly utilised lab technique is conventional PCR (Kim et al. 2001; Ishikawa et al. 1997) or real-time RT-PCR (Kim et al. 2007), which is sensitive, specific and rapid for the detection of viral RNA in different clinical samples. Although loop-mediated isothermal amplification (LAMP) assays (Ren and Li 2011; Yu et al. 2015) are recognised more recently as highly sensitive in detecting PEDV RNA, they have still not reached diagnostic labs. In situ hybridisation can be utilised to recognise PEDV RNA in fixed tissues (Stadler et al. 2015).
Direct display of PEDV and additionally its antigens is done utilising IF or IHC tests on the small intestinal tissues of piglets euthanised close to the onset of diarrhoea and before the desquamation of enterocytes. The virus particles can be shown in direct EM or IEM on diarrhoeic pig faeces. However, IEM must be used to distinguish PEDV from other CoVs, viz. TGEV and PDCoV, as all CoVs possess indistinguishable morphology.
Successful isolation of PEDV in Vero cells is increased when using intestinal contents/homogenates compared to faeces (Oka et al. 2014; Chen et al. 2014). ELISA tests based on polyclonal antibodies and MAbs are available for detection of PEDV antigens in faeces (Callebaut et al. 1982; Carvajal et al. 1995).
Paired serum samples are requisite for serologic diagnosis of endemic PEDV. Recently, IgG and IgA antibodies to PEDV are observed in oral fluids, suggesting that they may be appropriate to monitor prior herd exposure to PEDV (Bjustrom-Kraft et al. 2016). PEDV antibodies have been demonstrated using indirect ELISAs based on the whole cell-culture-adapted virus antigens (Carvajal et al. 1995; Hofmann and Wyler 1990; Kweon et al. 1994; Thomas et al. 2015), S/N viral proteins extracted from infected Vero cells (Knuchel et al. 1992; Oh et al. 2005) or bacteria or mammalian expression systems (Wang et al. 2015; Gerber et al. 2014; Gerber and Opriessnig 2015; Okda et al. 2015; Paudel et al. 2014). Blocking and competitive ELISAs have additionally been used for the detection of PEDV antibodies utilising MAbs or polyclonal antibodies (Carvajal et al. 1995; Okda et al. 2015; van Nieuwstadt and Zetstra 1991). Serum IgG antibodies against the N proteins of PEDV can be detected by 9–14 dpi; they peak around 21 dpi and then decline gradually (Okda et al. 2015). The VN test in Vero cell culture is used to assess VN antibodies to PEDV (Thomas et al. 2015; Okda et al. 2015; Paudel et al. 2014). These serological assays have generally been employed to screen prior exposure to the virus and to assess the viability of vaccines.
Overall, use of a laboratory test is suggested which must differentiate PEDV infections from TGEV, SeCoV and PDCoV. Especially for SeCoV that is a recombinant of TGEV (backbone) and PEDV (S protein), a selective assay based on detecting TGEV (any gene aside from S gene) and PEDV (S gene) would be preferred.
4.5.3 PDCoV
Selective laboratory assays must be opted to distinguish PDCoV infection from related PEDV, TGEV and RV infections. The methodologies discussed for TGEV and PEDV apply to PDCoV diagnosis. The confirmatory finding of PDCoV infection incorporates detection of PDCoV RNA or antigens in the faeces or intestinal substance/tissues. Diagnosis can also be made utilising RT-PCR assays that target a conserved region of PDCoV M or N genes (Marthaler et al. 2014b; Wang et al. 2014c), IF or IHC using virus-specific MAbs or polyclonal antibodies (Chen et al. 2015; Jung et al. 2015b; Ma et al. 2015), and in situ hybridisation (Jung et al. 2015b). A duplex real-time RT-PCR assay for detection of PDCoV and/or differentiation from PEDV in intestines and faeces was developed (Zhang et al. 2016b).
Direct EM can be used to display PDCoV viral particles in faeces from diarrhoeic pigs. However, IEM use must distinguish PDCoV from PEDV or TGEV (Jung et al. 2015a) using hyperimmune or convalescent sera. However isolation of PDCoV has been attempted with limited success in LLC-PK or ST cells, except in few strains (OH-FD22) (Hu et al. 2015). The other serologic assays in use for the diagnosis of PDCoV are IF, VN and ELISA. Serum and milk PDCoV antibodies of different isotypes have been quantified using ELISA based on cell culture virus antigen (Ma et al. 2016) or S1 and N viral proteins (Thachil et al. 2015; Okda et al. 2016; Su et al. 2016).
4.6 Transmission, Risk Factors and Stability
4.6.1 TGEV
TGEV is more stable in frozen conditions, but becomes fragile at room or higher temperatures. In an experimental study, the virus infectivity was maintained for more than 8 weeks at 5 °C, 2 weeks at 20 °C and 24 h at 35 °C in liquid manure slurry (Haas et al. 1995). Further, the virus is highly photosensitive and gets inactivated less than 6 h in the exposure of sunlight or ultraviolet light (Cartwright et al. 1965; Haelterman 1962). It also gets inactivated to 1% Lysovet (phenol and aldehyde), 0.03% formalin, 0.01% beta-propiolactone, 1 mM binary ethylenamine, sodium hypochlorite, NaOH, iodines, quaternary ammonium compounds, ether and chloroform (VanCott et al. 1993; Brown 1981). TGEV field strains are resistant to trypsin and relatively stable in pig bile, and at pH 3 (Laude et al. 1981), letting the virus survive in the stomach and small intestine.
In temperate climates, TGE is a seasonal disease with most outbreaks occurring in the winter months. It is assumed that this could be due to the virus stability in frozen form, and frailty to heat or sunlight (Haelterman 1962), permitting easy virus spread among herds in winter on fomites or animals. Three potential reservoirs for TGEV between seasonal epidemics proposed are (1) herds with enzootic TGE; (2) hosts other than swine and (3) carrier pigs. Dogs, cats and foxes could be the possible carriers, facilitating its spread on farms, as the shedding of the virus occurs for variable periods (Haelterman 1962; McClurkin et al. 1970) with the excreted virus (by dogs) remaining infectious for pigs (Haelterman 1962; Reynolds et al. 1980).
The increased concentration of wintering starlings (Sturnus vulgaris) in feedlots was suggested to contribute to the mechanical spread of TGEV between farms during the cold season. In a study, TGEV was seen in the droppings of starlings for as long as 32 h after feeding TGEV (Pilchard 1965). Likewise, housefly (Musca domestica) is also proposed as a mechanical vector for TGEV (Pilchard 1965). TGEV antigen is seen in flies on a swine herd and TGEV shedding up to 3 days from experimentally feeding flies (Gough and Jorgenson 1983). Notably, surveys done in Central Europe confirmed the presence of TGEV antibodies in nearly 30% of the feral pigs (Sedlak et al. 2008). Although TGEV shedding is detectable for up to 104 dpi (Underdahl et al. 1975), it is undefined yet whether infectious virus particles are shed or transmitted at that time. Adding of sentinel pigs to a herd at 3, 4 and 5 months after a past TGE outbreak resulted in no new disease in the introduced pigs (Derbyshire et al. 1969).
4.6.2 PRCV
Swine population density, farm distances and seasons influence PRCV epidemiology (Pensaert 1989; Have 1990). Pigs get PRCV infection at any age through contact or airborne transmission. The risk of spreading PRCV increases in zones of high swine density, where the virus can journey several miles.
4.6.3 PEDV
As for other enteric viral infections, in PEDV also direct or indirect faecal-oral transmission is the main route of virus transmission. Acute outbreaks in non-immune farms often occur within 4–5 days after newly purchased pig arrival. The virus enters farms mostly via infected pigs, but also by contaminated feed, trucks, boots or other fomites. Farm workers may also act as a vehicle for virus transmission to naïve pigs (Dee et al. 2014, 2016; Schumacher et al. 2016). Evidence of PEDV aerosol transmission is reported in some (Alonso et al. 2014) but not other studies (Niederwerder et al. 2016). In four-week-old pigs infected with emerging non-S INDEL PEDV strain, infectious virus excretion lasted for 14–16 days (Crawford et al. 2015). Nevertheless, a few pigs shed PEDV RNA, at 42 days post-initial oral exposure, but non-infectious virus particles were seen in faeces.
Similar to TGEV, after initial outbreaks on the breeding farms, PEDV can become endemic if sufficient litters of pigs are produced and weaned, allowing maintaining the virus. Of note, a report from South Korea showed 9.75% PEDV infection rate in wild boars (Lee et al. 2016b), although their role in maintenance and transmission of PEDV is unclear.
4.6.4 PDCoV
The main method of PDCoV transmission is the faecal–oral route. Faeces, vomit and other contaminated fomites are the major sources of the virus. Experimentally induced PDCoV diarrhoea lasted for ~5–10 days, with faecal virus RNA shedding lasting for up to 19 days (Hu et al. 2016; Ma et al. 2015). Pigs generally continue shedding PDCoV RNA in the faeces after recovery from disease; therefore, another possible reservoir for PDCoV may be subclinically infected or convalescent carriers.
4.7 Prevention and Control
4.7.1 TGEV
Treatment of clinically affected newborn piglets is usually ineffective in field situations; however, electrolyte/glucose solution supplementation of piglets that are 1 week or older may reduce their mortality (Bohl 1981). Extra heat, deep bedding and antibiotic solutions (to treat secondary infections) generally can improve piglet health.
Enhanced biosecurity measures should be maintained to decrease a chance of introduction of infected animals, and contaminated vehicles from TGEV-affected farms to susceptible herds. TGEV infection can be spread not only with infected live animals but also with unprocessed tissues of slaughtered TGEV-infected animals (Forman 1991).
Many methods for immunising sows to induce lactogenic immunity and consequent protection of neonatal piglets have been attempted (Chattha et al. 2015; Saif and Jackwood 1990; Bohl and Saif 1975). Several viral vaccines (virulent, attenuated, inactivated and recombinant subunit) with different routes of administration (oral, intra-nasal, subcutaneous, intramuscular and intra-mammary) (Saif and Sestak 2006; Moxley and Olson 1989) have been evaluated in the past. To note, intramuscular, parenteral or intra-mammary administration of pregnant sows with live attenuated, inactivated or subunit vaccines did not offer complete protection but were found to be effective to reduce piglet mortality rates (Brim et al. 1994). Unlike natural intestinal infection with the virulent virus, attenuated viruses do not stimulate the gut-MG-sIgA axis sufficiently for the induction of immunity similar to that observed following this. There are two commercial vaccines based on a live-modified TGEV strain for combined oral-intramuscular administration produced by Merck Animal Health: PROSYSTEM® TGE/Rota and PROSYSTEM® TREC. These vaccines can effectively stimulate a response in previously exposed pigs, but do not protect the naïve population.
Herd immunity can be enhanced by exposing all the sows to virulent TGEV (using intestinal contents or gut tissues of affected pigs) to boost lactogenic (milk) immunity (Bohl and Saif 1975; Bohl et al. 1972). This practice is called feedback and results in the rapid development of immunity in pregnant sows (particularly in those due to farrow 2 weeks or more after the start of the outbreak) and reduces losses in newborn piglets. However, it may also result in dissemination of other pathogens (potentially present in TGEV-containing faeces/intestinal contents) to adjacent herds. On small-scale farms, herd immunity is accomplished, and TGEV infection is self-limiting. In contrast, in larger farms (≤200 sows) with a continuous farrowing system and continual influx of susceptible animals, TGEV infection frequently becomes endemic after the primary outbreak (Saif and Sestak 2006). Elimination of endemic TGE in a herd can be attempted using the feedback method. After this is done, no weaning of piglets should occur during the following 3–4 weeks so that there remains no susceptible host in the herd while TGEV is circulating on the farm.
4.7.2 PEDV
Due to the lack of PEDV-specific antivirals, the treatment is focused on alleviating the diarrhoeal disease. PEDV-infected pigs must get enough water to reduce dehydration, which exacerbates the severity of the disease. Temporary withholding of feed may benefit fattening pigs during the acute stage of the disease.
As in the case with TGEV, appropriate biosecurity measures should be applied to avoid the introduction of PED onto farms. Present epidemiological knowledge indicates that virus is spread between farms mainly through animal and human traffic, and contaminated feed (https://www.aphis.usda.gov/animal_health/animal_dis_spec/swine/downloads/secd_final_report.pdf). Careful disposal of the dead stock is recommended.
In contrast to the present situation in Asia, in Europe, PEDV infection (with mostly mild S INDEL strains in circulation) is considered to be of marginal economic importance and therefore does not warrant the development of a vaccine (Lee 2015). However, severe classical PEDV outbreaks in Asia have necessitated the development of PEDV vaccines to prevent and control the infection. In China, the CV777-based inactivated and attenuated PEDV vaccines were approved in 1995 and 1998, respectively (Wang et al. 2016b). Soon after, attenuated vaccines based on classical PEDV strains KPEDV-9 and DR13 were commercialised in 1999 and 2004, respectively, in Korea (Kweon et al. 1999; Song et al. 2007). Since 1997 a commercial attenuated PEDV vaccine based on cell culture-adapted classical PEDV P-5V strain (Nisseiken Co. Ltd., Japan) is administered to sows in Japan (Sato et al. 2011). These vaccines based on classical PEDV strains appeared to satisfactorily control PED in Asia until the highly virulent non-S INDEL PEDV strains have emerged (Lee 2015). As demonstrated in the field, the classical PEDV vaccines have failed to protect pigs from severe diarrhoeal disease associated with the emerging highly virulent non-S INDEL PEDV strains (Lee 2015).
The deliberate exposure of pregnant sows (feedback method) to PEDV promotes the rapid development of lactogenic immunity and thus shortens the course and the severity of the disease on the farm (Chattha et al. 2015). However, as mentioned in the TGEV section, this method may contribute to the spread of other infectious agents throughout the farm. We have recently demonstrated that high dose of virulent PEDV administered to sows can substantially increase their piglet survival rate as compared to low-dose and mock-infected sows (Fig. 4.3) (Langel et al. 2016). This novel finding suggests that the current feedback-based control strategies can be improved by ensuring the uniform administration of high-dose PEDV to pregnant sows.
Mucosal immune responses and lactogenic immunity may be influenced by PEDV dose given to pregnant swine. Gilts received high and high, low PEDV dose or mock at 3–4 weeks prepartum. All piglets were PEDV-challenged at 3–5 days post-partum (Langel et al. 2016)
Since the outbreaks of 2013, the USA has conditionally licensed two PEDV vaccines targeting emerging non-S INDEL PEDV strains: alphavirus-based vaccine (Harris vaccines™, now Merck Animal Health) and an inactivated vaccine (Zoetis) (2014). The first vaccine was developed in June 2014 using a replication-deficient Venezuelan equine encephalitis (VEE) virus packaging system expressing the S protein of an emerging non-S INDEL PEDV strain (Crawford et al. 2016). The second vaccine developed in September 2015 was an inactivated whole-virus (non-S INDEL PEDV) vaccine plus an adjuvant (Crawford et al. 2016). In October 2016, an inactivated vaccine based on non-S INDEL PEDV strain AJ1102 was licensed in China (Wang et al. 2016b). In South Korea, an inactivated vaccine candidate based on non-S INDEL strain KNU-141112 was demonstrated to be protective in sows and their suckling piglets (Baek et al. 2016). However, the efficacy of these vaccines/vaccine candidates in the field is not assessed. To date, reverse genetics platforms have been generated for both classical and emerging non-S INDEL PEDV strains using different approaches (Beall et al. 2016; Jengarn et al. 2015; Li et al. 2013) and can be used for the future rational design of safe and effective PEDV vaccines.
4.7.3 PDCoV
The disease-preventive measures adopted for TGEV and PEDV control and prevention can be useful for PDCoV infection too. In the absence of any suitable vaccines or antivirals to control PDCoV disease, reliable regime includes symptomatic action giving bicarbonate liquids and ad lib water to alleviate acidosis and dehydration in suckling pigs. Antibiotics administration may be beneficial in the case complicated by concurrent/secondary bacterial infection. In the event of heavy mortality, feedback techniques must be opted to stimulate lactogenic immunity and reduce mortality. Additionally, during PDCoV epidemics, a strict biosecurity plan must be implemented to lessen PDCoV spread via infected fomites. The systems all in/all out and thorough disinfection (using phenolic disinfectants, bleach, peroxides, aldehydes or iodophors) can break the disease cycle.
References
Akimkin V, Beer M, Blome S et al (2016) New chimeric porcine coronavirus in swine feces, Germany, 2012. Emerg Infect Dis 22:1314–1315
Alonso C, Goede DP, Morrison RB et al (2014) Evidence of infectivity of airborne porcine epidemic diarrhea virus and detection of airborne viral RNA at long distances from infected herds. Vet Res 45:73
Annamalai T, Saif LJ, Lu Z et al (2015) Age-dependent variation in innate immune responses to porcine epidemic diarrhea virus infection in suckling versus weaned pigs. Vet Immunol Immunopathol 168:193–202
Anon (2014) USDA to require reports of PED. J Am Vet Med Assoc 244:1234
Atanasova K, Van Gucht S, Barbé F, Lefebvre DJ, Chiers K, Van Reeth K (2008) Lung cell tropism and inflammatory cytokine-profile of porcine respiratory coronavirus infection. Open Vet Sci J 2:117–126
Baek PS, Choi HW, Lee S et al (2016) Efficacy of an inactivated genotype 2b porcine epidemic diarrhea virus vaccine in neonatal piglets. Vet Immunol Immunopathol 174:45–49
Ballesteros ML, Sanchez CM, Enjuanes L (1997) Two amino acid changes at the N-terminus of transmissible gastroenteritis coronavirus spike protein result in the loss of enteric tropism. Virology 227:378–388
Barman N, Barman B, Sarma K et al (2003) Indian J Anim Sci 73:576–578
Beall A, Yount B, Lin CM et al (2016) Characterization of a pathogenic full-length cDNA clone and transmission model for porcine epidemic diarrhea virus strain PC22A. MBio 7:e01451-15
Belsham GJ, Rasmussen TB, Normann P et al (2016) Characterization of a novel chimeric swine enteric coronavirus from diseased pigs in Central Eastern Europe in 2016. Transbound Emerg Dis 63:595–601
Bernard S, Bottreau E, Aynaud JM et al (1989) Natural infection with the porcine respiratory coronavirus induces protective lactogenic immunity against transmissible gastroenteritis. Vet Microbiol 21:1–8
Bjustrom-Kraft J, Woodard K, Gimenez-Lirola L et al (2016) Porcine epidemic diarrhea virus (PEDV) detection and antibody response in commercial growing pigs. BMC Vet Res 12:99
Bohl E (1979) Diagnosis of diarrhea in pigs due to transmissible gastroenteritis virus or rotavirus. In: Bricout F, Scherrer R (eds) Viral enteritis in humans and animals. INSERM, Paris, pp 341–343
Bohl EH (1981) Transmissible gastroenteritis. In: Leman AD, Glock RD, Mengeling WL, Penny RHC, Scholl E, Straw B (eds) Diseases of swine, 5th edn. University Press, Ames, IA, pp 195–208
Bohl EH, Kumagai T (1965) The use of cell cultures for the study of swine transmissible gastroenteritis virus. In: United States Livestock Sanitary Association meeting
Bohl EH, Saif LJ (1975) Passive immunity in transmissible gastroenteritis of swine: immunoglobulin characteristics of antibodies in milk after inoculating virus by different routes. Infect Immun 11:23–32
Bohl EH, Gupta RK, Olquin MV et al (1972) Antibody responses in serum, colostrum, and milk of swine after infection or vaccination with transmissible gastroenteritis virus. Infect Immun 6:289–301
Bohl EH, Kohler EM, Saif LJ et al (1978) Rotavirus as a cause of diarrhea in pigs. J Am Vet Med Assoc 172:458–463
Boniotti MB, Papetti A, Lavazza A et al (2016) Porcine epidemic diarrhea virus and discovery of a recombinant swine enteric coronavirus, Italy. Emerg Infect Dis 22:83–87
Brim TA, VanCott JL, Lunney JK et al (1994) Lymphocyte proliferation responses of pigs inoculated with transmissible gastroenteritis virus or porcine respiratory coronavirus. Am J Vet Res 55:494–501
Brim TA, VanCott JL, Lunney JK et al (1995) Cellular immune responses of pigs after primary inoculation with porcine respiratory coronavirus or transmissible gastroenteritis virus and challenge with transmissible gastroenteritis virus. Vet Immunol Immunopathol 48:35–54
Brown I, Cartwright S (1986) New porcine coronavirus? Vet Rec 119:282–283
Brown TT Jr (1981) Laboratory evaluation of selected disinfectants as virucidal agents against porcine parvovirus, pseudorabies virus, and transmissible gastroenteritis virus. Am J Vet Res 42:1033–1036
Butler DG, Gall DG, Kelly MH et al (1974) Transmissible gastroenteritis. Mechanisms responsible for diarrhea in an acute viral enteritis in piglets. J Clin Invest 53:1335–1342
Callebaut P, Debouck P, Pensaert M (1982) Enzyme-linked immunosorbent assay for the detection of the coronavirus-like agent and its antibodies in pigs with porcine epidemic diarrhea. Vet Microbiol 7:295–306
Callebaut P, Pensaert MB, Hooyberghs J (1989) A competitive inhibition ELISA for the differentiation of serum antibodies from pigs infected with transmissible gastroenteritis virus (TGEV) or with the TGEV-related porcine respiratory coronavirus. Vet Microbiol 20:9–19
Cartwright SF, Harris HM, Blandford TB et al (1965) A cytopathic virus causing a transmissible gastroenteritis in swine. I. Isolation and properties. J Comp Pathol 75:387–396
Carvajal A, Lanza I, Diego R et al (1995) Evaluation of a blocking ELISA using monoclonal antibodies for the detection of porcine epidemic diarrhea virus and its antibodies. J Vet Diagn Investig 7:60–64
Cepica A, Derbyshire JB (1984) Antibody-dependent and spontaneous cell-mediated cytotoxicity against transmissible gastroenteritis virus infected cells by lymphocytes from sows, fetuses and neonatal piglets. Can J Comp Med 48:258–261
Chae C, Kim O, Choi C et al (2000) Prevalence of porcine epidemic diarrhoea virus and transmissible gastroenteritis virus infection in Korean pigs. Vet Rec 147:606–608
Chattha KS, Roth JA, Saif LJ (2015) Strategies for design and application of enteric viral vaccines. Annu Rev Anim Biosci 3:375–395
Chen W, Yan M, Yang L et al (2005) SARS-associated coronavirus transmitted from human to pig. Emerg Infect Dis 11:446–448
Chen Q, Li G, Stasko J et al (2014) Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak among swine in the United States. J Clin Microbiol 52:234–243
Chen Q, Gauger P, Stafne M et al (2015) Pathogenicity and pathogenesis of a United States porcine deltacoronavirus cell culture isolate in 5-day-old neonatal piglets. Virology 482:51–59
Chen Q, Gauger PC, Stafne MR et al (2016a) Pathogenesis comparison between the United States porcine epidemic diarrhea virus prototype and S-INDEL-variant strains in conventional neonatal piglets. J Gen Virol 97:1107–1121
Chen X, Tu C, Qin T et al (2016b) Retinoic acid facilitates inactivated transmissible gastroenteritis virus induction of CD8(+) T-cell migration to the porcine gut. Sci Rep 6:24152
Cheun-Arom T, Temeeyasen G, Srijangwad A et al (2015) Complete genome sequences of two genetically distinct variants of porcine epidemic diarrhea virus in the eastern region of Thailand. Genome Announc 3:e00634-15
Costantini V, Lewis P, Alsop J et al (2004) Respiratory and fecal shedding of porcine respiratory coronavirus (PRCV) in sentinel weaned pigs and sequence of the partial S-gene of the PRCV isolates. Arch Virol 149:957–974
Coussement W, Ducatelle R, Debouck P et al (1982) Pathology of experimental CV777 coronavirus enteritis in piglets. I. Histological and histochemical study. Vet Pathol 19:46–56
Cox E, Hooyberghs J, Pensaert MB (1990) Sites of replication of a porcine respiratory coronavirus related to transmissible gastroenteritis virus. Res Vet Sci 48:165–169
Cox E, Pensaert MB, Callebaut P (1993) Intestinal protection against challenge with transmissible gastroenteritis virus of pigs immune after infection with the porcine respiratory coronavirus. Vaccine 11:267–272
Crawford K, Lager K, Miller L et al (2015) Evaluation of porcine epidemic diarrhea virus transmission and the immune response in growing pigs. Vet Res 46:49
Crawford K, Lager KM, Kulshreshtha V et al (2016) Status of vaccines for porcine epidemic diarrhea virus in the United States and Canada. Virus Res 226:108–116
De Diego M, Laviada MD, Enjuanes L et al (1992) Epitope specificity of protective lactogenic immunity against swine transmissible gastroenteritis virus. J Virol 66:6502–6508
de Groot R, Ziebuhr J, Poon L et al (2008) Revision of the family Coronaviridae. Taxonomic proposal of the Coronavirus Study Group to the ICTV Executive Committee. http://talk.ictvonline.org/media/p/1230.aspx
Debouck P, Pensaert M, Coussement W (1981) The pathogenesis of an enteric infection in pigs, experimentally induced by the coronavirus-like agent, CV 777. Vet Microbiol 6:157–165
Dee S, Clement T, Schelkopf A et al (2014) An evaluation of contaminated complete feed as a vehicle for porcine epidemic diarrhea virus infection of naive pigs following consumption via natural feeding behavior: proof of concept. BMC Vet Res 10:176
Dee S, Neill C, Singrey A et al (2016) Modeling the transboundary risk of feed ingredients contaminated with porcine epidemic diarrhea virus. BMC Vet Res 12:51
Derbyshire JB, Jessett DM, Newman G (1969) An experimental epidemiological study of porcine transmissible gastroenteritis. J Comp Pathol 79:445–452
Diep NV, Norimine J, Sueyoshi M et al (2017) Novel porcine epidemic diarrhea virus (PEDV) variants with large deletions in the spike (S) gene coexist with PEDV strains possessing an intact S gene in domestic pigs in Japan: a new disease situation. PLoS One 12:e0170126
Ding Z, Fang L, Jing H et al (2014) Porcine epidemic diarrhea virus nucleocapsid protein antagonizes beta interferon production by sequestering the interaction between IRF3 and TBK1. J Virol 88:8936–8945
Dong BQ, Liu W, Fan XH et al (2007) Detection of a novel and highly divergent coronavirus from Asian leopard cats and Chinese ferret badgers in southern China. J Virol 81:6920–6926
Dong N, Fang L, Zeng S et al (2015) Porcine deltacoronavirus in mainland China. Emerg Infect Dis 21:2254–2255
Doyle LP, Hutchings LM (1946) A transmissible gastroenteritis in pigs. J Am Vet Med Assoc 108:257–259
Enjuanes L, Van der Zeijst B (1995) Molecular basis of transmissible gastroenteritis virus epidemiology. In: Siddell SG (ed) The Coronaviridae. Plenum Press, New York, pp 337–376
Forman AJ (1991) Infection of pigs with transmissible gastroenteritis virus from contaminated carcases. Aust Vet J 68:25–27
Frederick GT, Bohl EH, Cross RF (1976) Pathogenicity of an attenuated strain of transmissible gastroenteritis virus for newborn pigs. Am J Vet Res 37:165–169
Garwes DJ, Stewart F, Cartwright SF et al (1988) Differentiation of porcine coronavirus from transmissible gastroenteritis virus. Vet Rec 122:86–87
Gerber PF, Opriessnig T (2015) Detection of immunoglobulin (Ig) A antibodies against porcine epidemic diarrhea virus (PEDV) in fecal and serum samples. MethodsX 2:368–373
Gerber PF, Gong Q, Huang YW et al (2014) Detection of antibodies against porcine epidemic diarrhea virus in serum and colostrum by indirect ELISA. Vet J 202:33–36
Gong L, Li J, Zhou Q et al (2017) A new bat-HKU2-like coronavirus in swine, China, 2017. Emerg Infect Dis 23. https://doi.org/10.3201/eid2309.170915
Gonzalez JM, Gomez-Puertas P, Cavanagh D et al (2003) A comparative sequence analysis to revise the current taxonomy of the family Coronaviridae. Arch Virol 148:2207–2235
Gough PM, Jorgenson RD (1983) Identification of porcine transmissible gastroenteritis virus in house flies (Musca domestica Linneaus). Am J Vet Res 44:2078–2082
Haas B, Ahl R, Bohm R et al (1995) Inactivation of viruses in liquid manure. Rev Sci Tech 14:435–445
Haelterman EO (1962) Epidemiological studies of transmissible gastroenteritis of swine. In: United States Livestock Sanitary Association meeting
Halbur PG, Paul PS, Vaughn EM et al (1993) Experimental reproduction of pneumonia in gnotobiotic pigs with porcine respiratory coronavirus isolate AR310. J Vet Diagn Investig 5:184–188
Have P (1990) Infection with a new porcine respiratory coronavirus in Denmark: serologic differentiation from transmissible gastroenteritis virus using monoclonal antibodies. Adv Exp Med Biol 276:435–439
Hill H, Biwer J, Woods R, Wesley R (1990) Porcine respiratory coronavirus isolated from two U.S. swine herds. In: American Association of Swine Practitioners meeting
Hofmann M, Wyler R (1988) Propagation of the virus of porcine epidemic diarrhea in cell culture. J Clin Microbiol 26:2235–2239
Hofmann M, Wyler R (1990) Enzyme-linked immunosorbent assay for the detection of porcine epidemic diarrhea coronavirus antibodies in swine sera. Vet Microbiol 21:263–273
Hooper BE, Haelterman EO (1966a) Concepts of pathogenesis and passive immunity in transmissible gastroenteritis of swine. J Am Vet Med Assoc 149:1580–1586
Hooper BE, Haelterman EO (1966b) Growth of transmissible gastroenteritis virus in young pigs. Am J Vet Res 27:286–291
Hou Y, Lin CM, Yokoyama M et al (2017) Deletion of a 197-amino-acid region in the N-terminal domain of spike protein attenuates porcine epidemic diarrhea virus in piglets. J Virol 91:e00227-17
Hu H, Jung K, Vlasova AN et al (2015) Isolation and characterization of porcine deltacoronavirus from pigs with diarrhea in the United States. J Clin Microbiol 53:1537–1548
Hu H, Jung K, Vlasova AN et al (2016) Experimental infection of gnotobiotic pigs with the cell-culture-adapted porcine deltacoronavirus strain OH-FD22. Arch Virol 161:3421–3434
Hwang EK, Kim JH, Jean YH et al (1994) Current occurrence of porcine epidemic diarrhea in Korea. RDA J Agric Sci 36:587–596
Ishikawa K, Sekiguchi H, Ogino T et al (1997) Direct and rapid detection of porcine epidemic diarrhea virus by RT-PCR. J Virol Methods 69:191–195
Janetanakit T, Lumyai M, Bunpapong N et al (2016) Porcine deltacoronavirus, Thailand, 2015. Emerg Infect Dis 22:757–759
Jengarn J, Wongthida P, Wanasen N et al (2015) Genetic manipulation of porcine epidemic diarrhea virus (PEDV) recovered from a full length infectious cDNA clone. J Gen Virol 96:2206–2218
Jung K, Alekseev KP, Zhang X et al (2007) Altered pathogenesis of porcine respiratory coronavirus in pigs due to immunosuppressive effects of dexamethasone: implications for corticosteroid use in treatment of severe acute respiratory syndrome coronavirus. J Virol 81:13681–13693
Jung K, Wang Q, Scheuer KA et al (2014) Pathology of US porcine epidemic diarrhea virus strain PC21A in gnotobiotic pigs. Emerg Infect Dis 20:662–665
Jung K, Annamalai T, Lu Z et al (2015a) Comparative pathogenesis of US porcine epidemic diarrhea virus (PEDV) strain PC21A in conventional 9-day-old nursing piglets vs. 26-day-old weaned pigs. Vet Microbiol 178:31–40
Jung K, Hu H, Eyerly B et al (2015b) Pathogenicity of 2 porcine deltacoronavirus strains in gnotobiotic pigs. Emerg Infect Dis 21:650–654
Jung K, Hu H, Saif LJ (2016a) Porcine deltacoronavirus induces apoptosis in swine testicular and LLC porcine kidney cell lines in vitro but not in infected intestinal enterocytes in vivo. Vet Microbiol 182:57–63
Jung K, Hu H, Saif LJ (2016b) Porcine deltacoronavirus infection: etiology, cell culture for virus isolation and propagation, molecular epidemiology and pathogenesis. Virus Res 226:50–59
Kemeny LJ, Wiltsey VL, Riley JL (1975) Upper respiratory infection of lactating sows with transmissible gastroenteritis virus following contact exposure to infected piglets. Cornell Vet 65:352–362
Kim O, Chae C (2003) Experimental infection of piglets with a Korean strain of porcine epidemic diarrhoea virus. J Comp Pathol 129:55–60
Kim L, Chang KO, Sestak K et al (2000) Development of a reverse transcription-nested polymerase chain reaction assay for differential diagnosis of transmissible gastroenteritis virus and porcine respiratory coronavirus from feces and nasal swabs of infected pigs. J Vet Diagn Investig 12:385–388
Kim SY, Song DS, Park BK (2001) Differential detection of transmissible gastroenteritis virus and porcine epidemic diarrhea virus by duplex RT-PCR. J Vet Diagn Investig 13:516–520
Kim SH, Kim IJ, Pyo HM et al (2007) Multiplex real-time RT-PCR for the simultaneous detection and quantification of transmissible gastroenteritis virus and porcine epidemic diarrhea virus. J Virol Methods 146:172–177
Kim SH, Lee JM, Jung J et al (2015) Genetic characterization of porcine epidemic diarrhea virus in Korea from 1998 to 2013. Arch Virol 160:1055–1064
Kim YK, Cho YY, An BH et al (2016) Molecular characterization of the spike and ORF3 genes of porcine epidemic diarrhea virus in the Philippines. Arch Virol 161:1323–1328
Knuchel M, Ackermann M, Muller HK et al (1992) An ELISA for detection of antibodies against porcine epidemic diarrhoea virus (PEDV) based on the specific solubility of the viral surface glycoprotein. Vet Microbiol 32:117–134
Kodama Y, Ogata M, Simizu Y (1980) Characterization of immunoglobulin A antibody in serum of swine inoculated with transmissible gastroenteritis virus. Am J Vet Res 41:740–745
Ksiazek TG, Erdman D, Goldsmith CS et al (2003) A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 348:1953–1966
Kuwahara H, Nunoya T, Samejima T et al (1988) Passage in piglets of a coronavirus associated with porcine epidemic diarrhea. J Jpn Vet Med Assoc 41:169–173
Kweon C, Kwon BJ, Jung TS et al (1993) Isolation of porcine epidemic diarrhea virus (PEDV) infection in Korea. Korean J Vet Res 33:249–254
Kweon CH, Kwon BJ, Kang YB et al (1994) Cell adaptation of KPEDV-9 and serological survey on porcine epidemic diarrhea virus (PEDV) infection in Korea. Korean J Vet Res 34:321–326
Kweon CH, Kwon BJ, Lee JG et al (1999) Derivation of attenuated porcine epidemic diarrhea virus (PEDV) as vaccine candidate. Vaccine 17:2546–2553
Langel SN, Paim FC, Lager KM et al (2016) Lactogenic immunity and vaccines for porcine epidemic diarrhea virus (PEDV): historical and current concepts. Virus Res 226:93–107
Lanza I, Shoup DI, Saif LJ (1995) Lactogenic immunity and milk antibody isotypes to transmissible gastroenteritis virus in sows exposed to porcine respiratory coronavirus during pregnancy. Am J Vet Res 56:739–748
Laude H, Gelfi J, Aynaud JM (1981) In vitro properties of low- and high-passaged strains of transmissible gastroenteritis coronavirus of swine. Am J Vet Res 42:447–449
Laude H, Van Reeth K, Pensaert M (1993) Porcine respiratory coronavirus: molecular features and virus-host interactions. Vet Res 24:125–150
Lee C (2015) Porcine epidemic diarrhea virus: an emerging and re-emerging epizootic swine virus. Virol J 12:193
Lee S, Park GS, Shin JH et al (2014) Full-genome sequence analysis of a variant strain of porcine epidemic diarrhea virus in South Korea. Genome Announc 2:e00753-14
Lee JH, Chung HC, Nguyen VG et al (2016a) Detection and phylogenetic analysis of porcine deltacoronavirus in Korean swine farms, 2015. Transbound Emerg Dis 63:248–252
Lee DU, Kwon T, Je SH et al (2016b) Wild boars harboring porcine epidemic diarrhea virus (PEDV) may play an important role as a PEDV reservoir. Vet Microbiol 192:90–94
Li C, Li Z, Zou Y et al (2013) Manipulation of the porcine epidemic diarrhea virus genome using targeted RNA recombination. PLoS One 8:e69997
Li G, Chen Q, Harmon KM et al (2014) Full-length genome sequence of porcine deltacoronavirus strain USA/IA/2014/8734. Genome Announc 2:e00278-14
Li Z, He W, Lan Y et al (2016) The evidence of porcine hemagglutinating encephalomyelitis virus induced nonsuppurative encephalitis as the cause of death in piglets. PeerJ 4:e2443
Lin CN, Chung WB, Chang SW et al (2014) US-like strain of porcine epidemic diarrhea virus outbreaks in Taiwan, 2013-2014. J Vet Med Sci 76:1297–1299
Lin CM, Gao X, Oka T et al (2015a) Antigenic relationships among porcine epidemic diarrhea virus and transmissible gastroenteritis virus strains. J Virol 89:3332–3342
Lin CM, Annamalai T, Liu X et al (2015b) Experimental infection of a US spike-insertion deletion porcine epidemic diarrhea virus in conventional nursing piglets and cross-protection to the original US PEDV infection. Vet Res 46:134
Lin CM, Saif LJ, Marthaler D et al (2016) Evolution, antigenicity and pathogenicity of global porcine epidemic diarrhea virus strains. Virus Res 226:20–39
Lohse L, Krog JS, Strandbygaard B et al (2017) Experimental infection of young pigs with an early European strain of porcine epidemic diarrhoea virus and a recent US strain. Transbound Emerg Dis 64:1380–1386
Lorbach JN, Wang L, Nolting JM et al (2017) Porcine hemagglutinating encephalomyelitis virus and respiratory disease in exhibition swine, Michigan, USA, 2015. Emerg Infect Dis 23:1168–1171
Ma G, Feng Y, Gao F et al (2005) Biochemical and biophysical characterization of the transmissible gastroenteritis coronavirus fusion core. Biochem Biophys Res Commun 337:1301–1307
Ma Y, Zhang Y, Liang X et al (2015) Origin, evolution, and virulence of porcine deltacoronaviruses in the United States. MBio 6:e00064
Ma Y, Zhang Y, Liang X et al (2016) Two-way antigenic cross-reactivity between porcine epidemic diarrhea virus and porcine deltacoronavirus. Vet Microbiol 186:90–96
Madson DM, Magstadt DR, Arruda PH et al (2014) Pathogenesis of porcine epidemic diarrhea virus isolate (US/Iowa/18984/2013) in 3-week-old weaned pigs. Vet Microbiol 174:60–68
Martelli P, Lavazza A, Nigrelli AD et al (2008) Epidemic of diarrhoea caused by porcine epidemic diarrhoea virus in Italy. Vet Rec 162:307–310
Marthaler D, Jiang Y, Collins J et al (2014a) Complete genome sequence of strain SDCV/USA/Illinois121/2014, a porcine deltacoronavirus from the United States. Genome Announc 2:e00218-14
Marthaler D, Raymond L, Jiang Y et al (2014b) Rapid detection, complete genome sequencing, and phylogenetic analysis of porcine deltacoronavirus. Emerg Infect Dis 20:1347–1350
Masuda T, Murakami S, Takahashi O et al (2015) New porcine epidemic diarrhoea virus variant with a large deletion in the spike gene identified in domestic pigs. Arch Virol 160:2565–2568
Masuda T, Tsuchiaka S, Ashiba T et al (2016) Development of one-step real-time reverse transcriptase-PCR-based assays for the rapid and simultaneous detection of four viruses causing porcine diarrhea. Jpn J Vet Res 64:5–14
McClurkin AW, Norman JO (1966) Studies on transmissible gastroenteritis of swine. II. Selected characteristics of a cytopathogenic virus common to five isolates from transmissible gastroenteritis. Can J Comp Med Vet Sci 30:190–198
McClurkin AW, Stark SL, Norman JO (1970) Transmissible gastroenteritis (TGE) of swine: the possible role of dogs in the epizootiology of TGE. Can J Comp Med 34:347–349
Moon HW (1978) Mechanisms in the pathogenesis of diarrhea: a review. J Am Vet Med Assoc 172:443–448
Moon HW, Kemeny LJ, Lambert G et al (1975) Age-dependent resistance to transmissible gastroenteritis of swine. III. Effects of epithelial cell kinetics on coronavirus production and on atrophy of intestinal villi. Vet Pathol 12:434–445
Moxley RA, Olson LD (1989) Clinical evaluation of transmissible gastroenteritis virus vaccines and vaccination procedures for inducing lactogenic immunity in sows. Am J Vet Res 50:111–118
Niederwerder MC, Nietfeld JC, Bai J et al (2016) Tissue localization, shedding, virus carriage, antibody response, and aerosol transmission of porcine epidemic diarrhea virus following inoculation of 4-week-old feeder pigs. J Vet Diagn Investig 28:671–678
Ogawa H, Taira O, Hirai T et al (2009) Multiplex PCR and multiplex RT-PCR for inclusive detection of major swine DNA and RNA viruses in pigs with multiple infections. J Virol Methods 160:210–214
Oh JS, Song DS, Yang JS et al (2005) Comparison of an enzyme-linked immunosorbent assay with serum neutralization test for serodiagnosis of porcine epidemic diarrhea virus infection. J Vet Sci 6:349–352
Oka T, Saif LJ, Marthaler D et al (2014) Cell culture isolation and sequence analysis of genetically diverse US porcine epidemic diarrhea virus strains including a novel strain with a large deletion in the spike gene. Vet Microbiol 173:258–269
Okda F, Liu X, Singrey A et al (2015) Development of an indirect ELISA, blocking ELISA, fluorescent microsphere immunoassay and fluorescent focus neutralization assay for serologic evaluation of exposure to North American strains of porcine epidemic diarrhea virus. BMC Vet Res 11:180
Okda F, Lawson S, Liu X et al (2016) Development of monoclonal antibodies and serological assays including indirect ELISA and fluorescent microsphere immunoassays for diagnosis of porcine deltacoronavirus. BMC Vet Res 12:95
O’Toole D, Brown I, Bridges A et al (1989) Pathogenicity of experimental infection with ‘pneumotropic’ porcine coronavirus. Res Vet Sci 47:23–29
Park JE, Shin HJ (2014) Porcine epidemic diarrhea virus infects and replicates in porcine alveolar macrophages. Virus Res 191:143–152
Paudel S, Park JE, Jang H et al (2014) Comparison of serum neutralization and enzyme-linked immunosorbent assay on sera from porcine epidemic diarrhea virus vaccinated pigs. Vet Q 34:218–223
PED vaccine gains conditional approval. J Am Vet Med Assoc 2014;245:267
Pensaert M (1989) Transmissible gastroenteritis virus (respiratory variant). In: Pensaert M (ed) Virus infections of porcines, vol 2. Elsevier Science Publishers, Amsterdam, pp 154–165
Pensaert M, Haelterman EO, Burnstein T (1970) Transmissible gastroenteritis of swine: virus-intestinal cell interactions. I. Immunofluorescence, histopathology and virus production in the small intestine through the course of infection. Arch Gesamte Virusforsch 31:321–334
Pensaert M, Callebaut P, Vergote J (1986) Isolation of a porcine respiratory, non-enteric coronavirus related to transmissible gastroenteritis. Vet Q 8:257–261
Pensaert M, Cox E, van Deun K et al (1993) A sero-epizootiological study of porcine respiratory coronavirus in Belgian swine. Vet Q 15:16–20
Pilchard EI (1965) Experimental transmission of transmissible gastroenteritis virus by starlings. Am J Vet Res 26:1177–1179
Pospischil A, Hess RG, Bachmann PA (1981) Light microscopy and ultrahistology of intestinal changes in pigs infected with epizootic diarrhoea virus (EVD): comparison with transmissible gastroenteritis (TGE) virus and porcine rotavirus infections. Zentralbl Veterinarmed B 28:564–577
Pritchard GC (1987) Transmissible gastroenteritis in endemically infected breeding herds of pigs in East Anglia, 1981-85. Vet Rec 120:226–230
Puranaveja S, Poolperm P, Lertwatcharasarakul P et al (2009) Chinese-like strain of porcine epidemic diarrhea virus, Thailand. Emerg Infect Dis 15:1112–1115
Ren X, Li P (2011) Development of reverse transcription loop-mediated isothermal amplification for rapid detection of porcine epidemic diarrhea virus. Virus Genes 42:229–235
Reynolds DJ, Garwes DJ, Lucey S (1980) Differentiation of canine coronavirus and porcine transmissible gastroenteritis virus by neutralization with canine, porcine, and feline sera. Vet Microbiol 5:283–290
Saeng-Chuto K, Lorsirigool A, Temeeyasen G et al (2017) Different lineage of porcine deltacoronavirus in Thailand, Vietnam and Lao PDR in 2015. Transbound Emerg Dis 64:3–10
Saif LJ (1999) Enteric viral infections of pigs and strategies for induction of mucosal immunity. Adv Vet Med 41:429–446
Saif LJ, Bohl EH (1979) Role of SIgA in passive immunity of swine to enteric viral infections. In: Ogra P, Dayton D (eds) Immunology of breast milk. Raven Press, New York, NY, pp 237–248
Saif L, Jackwood DJ (1990) Enteric virus vaccines: theoretical considerations, current status, and future approaches. In: Saif L, Theil KW (eds) Viral diarrheas of man and animals. CRC Press, Inc., Boca Raton, FL, pp 313–329
Saif LJ, Sestak K (2006) Transmissible gastroenteritis virus and porcine respiratory coronavirus. In: Straw BE, Zimmerman JJ, D’Allaire D, Taylor DJ (eds) Diseases of swine, 9th edn. Blackwell Publishing Company, Ames, IA, pp 489–516
Saif LJ, Bohl EH, Gupta RK (1972) Isolation of porcine immunoglobulins and determination of the immunoglobulin classes of transmissible gastroenteritis viral antibodies. Infect Immun 6:600–609
Saif LJ, Bohl EH, Kohler EM et al (1977) Immune electron microscopy of transmissible gastroenteritis virus and rotavirus (reovirus-like agent) of swine. Am J Vet Res 38:13–20
Saif LJ, van Cott JL, Brim TA (1994) Immunity to transmissible gastroenteritis virus and porcine respiratory coronavirus infections in swine. Vet Immunol Immunopathol 43:89–97
Sanchez CM, Izeta A, Sanchez-Morgado JM et al (1999) Targeted recombination demonstrates that the spike gene of transmissible gastroenteritis coronavirus is a determinant of its enteric tropism and virulence. J Virol 73:7607–7618
Sato T, Takeyama N, Katsumata A et al (2011) Mutations in the spike gene of porcine epidemic diarrhea virus associated with growth adaptation in vitro and attenuation of virulence in vivo. Virus Genes 43:72–78
Schumacher LL, Woodworth JC, Jones CK et al (2016) Evaluation of the minimum infectious dose of porcine epidemic diarrhea virus in virus-inoculated feed. Am J Vet Res 77:1108–1113
Sedlak K, Bartova E, Machova J (2008) Antibodies to selected viral disease agents in wild boars from the Czech Republic. J Wildl Dis 44:777–780
Sestak K, Lanza I, Park SK et al (1996) Contribution of passive immunity to porcine respiratory coronavirus to protection against transmissible gastroenteritis virus challenge exposure in suckling pigs. Am J Vet Res 57:664–671
Sestak K, Zhou Z, Shoup DI et al (1999a) Evaluation of the baculovirus-expressed S glycoprotein of transmissible gastroenteritis virus (TGEV) as antigen in a competition ELISA to differentiate porcine respiratory coronavirus from TGEV antibodies in pigs. J Vet Diagn Investig 11:205–214
Sestak K, Meister RK, Hayes JR et al (1999b) Active immunity and T-cell populations in pigs intraperitoneally inoculated with baculovirus-expressed transmissible gastroenteritis virus structural proteins. Vet Immunol Immunopathol 70:203–221
Shimizu M, Shimizu Y (1979) Lymphocyte proliferative response to viral antigen in pigs infected with transmissible gastroenteritis virus. Infect Immun 23:239–243
Shoup DI, Swayne DE, Jackwood DJ et al (1996) Immunohistochemistry of transmissible gastroenteritis virus antigens in fixed paraffin-embedded tissues. J Vet Diagn Investig 8:161–167
Simkins RA, Weilnau PA, Van Cott J et al (1993) Competition ELISA, using monoclonal antibodies to the transmissible gastroenteritis virus (TGEV) S protein, for serologic differentiation of pigs infected with TGEV or porcine respiratory coronavirus. Am J Vet Res 54:254–259
Sinha A, Gauger P, Zhang J et al (2015) PCR-based retrospective evaluation of diagnostic samples for emergence of porcine deltacoronavirus in US swine. Vet Microbiol 179:296–298
Song DS, Oh JS, Kang BK et al (2007) Oral efficacy of Vero cell attenuated porcine epidemic diarrhea virus DR13 strain. Res Vet Sci 82:134–140
Song D, Zhou X, Peng Q et al (2015) Newly emerged porcine deltacoronavirus associated with diarrhoea in swine in China: identification, prevalence and full-length genome sequence analysis. Transbound Emerg Dis 62:575–580
Stadler J, Zoels S, Fux R et al (2015) Emergence of porcine epidemic diarrhea virus in southern Germany. BMC Vet Res 11:142
Stepanek J, Mensik J, Franz J, Hornich M (1979) Epizootiology, diagnosis and prevention of viral diarrhea in piglets under intensive husbandry conditions. In: 21st World Veterinary congress, Moscow
Stevenson GW, Hoang H, Schwartz KJ et al (2013) Emergence of porcine epidemic diarrhea virus in the United States: clinical signs, lesions, and viral genomic sequences. J Vet Diagn Investig 25:649–654
Stone SS, Kemeny LJ, Woods RD et al (1977) Efficacy of isolated colostral IgA, IgG, and IgM(A) to protect neonatal pigs against the coronavirus of transmissible gastroenteritis. Am J Vet Res 38:1285–1288
Su M, Li C, Guo D et al (2016) A recombinant nucleocapsid protein-based indirect enzyme-linked immunosorbent assay to detect antibodies against porcine deltacoronavirus. J Vet Med Sci 78:601–606
Sueyoshi M, Tsuda T, Yamazaki K et al (1995) An immunohistochemical investigation of porcine epidemic diarrhoea. J Comp Pathol 113:59–67
Sun RQ, Cai RJ, Chen YQ et al (2012) Outbreak of porcine epidemic diarrhea in suckling piglets, China. Emerg Infect Dis 18:161–163
Sun D, Wang X, Wei S et al (2016) Epidemiology and vaccine of porcine epidemic diarrhea virus in China: a mini-review. J Vet Med Sci 78:355–363
Suzuki T, Murakami S, Takahashi O et al (2015) Molecular characterization of pig epidemic diarrhoea viruses isolated in Japan from 2013 to 2014. Infect Genet Evol 36:363–368
Suzuki T, Shibahara T, Yamaguchi R et al (2016) Pig epidemic diarrhoea virus S gene variant with a large deletion non-lethal to colostrum-deprived newborn piglets. J Gen Virol 97:1823–1828
Takahashi K, Okada K, Ohshima K (1983) An outbreak of swine diarrhea of a new-type associated with coronavirus-like particles in Japan. Nihon Juigaku Zasshi 45:829–832
Thachil A, Gerber PF, Xiao CT et al (2015) Development and application of an ELISA for the detection of porcine deltacoronavirus IgG antibodies. PLoS One 10:e0124363
Thomas JT, Chen Q, Gauger PC et al (2015) Effect of porcine epidemic diarrhea virus infectious doses on infection outcomes in naive conventional neonatal and weaned pigs. PLoS One 10:e0139266
Underdahl NR, Mebus CA, Torres-Medina A (1975) Recovery of transmissible gastroenteritis virus from chronically infected experimental pigs. Am J Vet Res 36:1473–1476
van Nieuwstadt AP, Zetstra T (1991) Use of two enzyme-linked immunosorbent assays to monitor antibody responses in swine with experimentally induced infection with porcine epidemic diarrhea virus. Am J Vet Res 52:1044–1050
van Nieuwstadt AP, Cornelissen JB, Zetstra T (1988) Comparison of two methods for detection of transmissible gastroenteritis virus in feces of pigs with experimentally induced infection. Am J Vet Res 49:1836–1843
van Nieuwstadt AP, Zetstra T, Boonstra J (1989) Infection with porcine respiratory coronavirus does not fully protect pigs against intestinal transmissible gastroenteritis virus. Vet Rec 125:58–60
VanCott JL, Brim TA, Simkins RA et al (1993) Isotype-specific antibody-secreting cells to transmissible gastroenteritis virus and porcine respiratory coronavirus in gut- and bronchus-associated lymphoid tissues of suckling pigs. J Immunol 150:3990–4000
VanCott JL, Brim TA, Lunney JK et al (1994) Contribution of antibody-secreting cells induced in mucosal lymphoid tissues of pigs inoculated with respiratory or enteric strains of coronavirus to immunity against enteric coronavirus challenge. J Immunol 152:3980–3990
Vlasova AN, Marthaler D, Wang Q et al (2014) Distinct characteristics and complex evolution of PEDV strains, North America, May 2013–February 2014. Emerg Infect Dis 20:1620–1628
Vui DT, Tung N, Inui K et al (2014) Complete genome sequence of porcine epidemic diarrhea virus in Vietnam. Genome Announc 2
Wang L, Byrum B, Zhang Y (2014a) New variant of porcine epidemic diarrhea virus, United States, 2014. Emerg Infect Dis 20:917–919
Wang L, Byrum B, Zhang Y (2014b) Detection and genetic characterization of deltacoronavirus in pigs, Ohio, USA, 2014. Emerg Infect Dis 20:1227–1230
Wang L, Byrum B, Zhang Y (2014c) Porcine coronavirus HKU15 detected in 9 US states, 2014. Emerg Infect Dis 20:1594–1595
Wang D, Fang L, Shi Y et al (2015) Porcine epidemic diarrhea virus 3C-like protease regulates its interferon antagonism by cleaving NEMO. J Virol 90:2090–2101
Wang L, Hayes J, Sarver C et al (2016a) Porcine deltacoronavirus: histological lesions and genetic characterization. Arch Virol 161:171–175
Wang D, Fang L, Xiao S (2016b) Porcine epidemic diarrhea in China. Virus Res 226:7–13
Wesley RD, Woods RD (1996) Induction of protective immunity against transmissible gastroenteritis virus after exposure of neonatal pigs to porcine respiratory coronavirus. Am J Vet Res 57:157–162
Wesley RD, Woods RD, Hill HT et al (1990) Evidence for a porcine respiratory coronavirus, antigenically similar to transmissible gastroenteritis virus, in the United States. J Vet Diagn Investig 2:312–317
Wesley RD, Woods RD, McKean JD et al (1997) Prevalence of coronavirus antibodies in Iowa swine. Can J Vet Res 61:305–308
Witte KH (1971) Isolation of the virus of transmissible gastroenteritis (TGE) from naturally infected piglets in cell culture. Zentralbl Veterinarmed B 18:770–778
Woo PC, Lau SK, Lam CS et al (2012) Discovery of seven novel mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J Virol 86:3995–4008
Xuan H, Xing D, Wang D et al (1984) Culture of porcine epidemic diarrhea virus by using pig intestinal monolayer cell. J People’s Liber Army Vet Univ 4:202–208
Yaeger M, Funk N, Hoffman L (2002) A survey of agents associated with neonatal diarrhea in Iowa swine including Clostridium difficile and porcine reproductive and respiratory syndrome virus. J Vet Diagn Investig 14:281–287
Yu X, Shi L, Lv X et al (2015) Development of a real-time reverse transcription loop-mediated isothermal amplification method for the rapid detection of porcine epidemic diarrhea virus. Virol J 12:76
Zhang J (2016) Porcine deltacoronavirus: overview of infection dynamics, diagnostic methods, prevalence and genetic evolution. Virus Res 226:71–84
Zhang Q, Shi K, Yoo D (2016a) Suppression of type I interferon production by porcine epidemic diarrhea virus and degradation of CREB-binding protein by nsp1. Virology 489:252–268
Zhang J, Tsai YL, Lee PY et al (2016b) Evaluation of two singleplex reverse transcription-insulated isothermal PCR tests and a duplex real-time RT-PCR test for the detection of porcine epidemic diarrhea virus and porcine deltacoronavirus. J Virol Methods 234:34–42
Zhao S, Gao Q, Qin T et al (2014) Effects of virulent and attenuated transmissible gastroenteritis virus on the ability of porcine dendritic cells to sample and present antigen. Vet Microbiol 171:74–86
Acknowledgements
All the authors of the manuscript thank and acknowledge their respective universities and institutes.
Conflict of interest: There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Vlasova, A.N., Wang, Q., Jung, K., Langel, S.N., Malik, Y.S., Saif, L.J. (2020). Porcine Coronaviruses. In: Malik, Y., Singh, R., Yadav, M. (eds) Emerging and Transboundary Animal Viruses . Livestock Diseases and Management. Springer, Singapore. https://doi.org/10.1007/978-981-15-0402-0_4
Download citation
DOI: https://doi.org/10.1007/978-981-15-0402-0_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-0401-3
Online ISBN: 978-981-15-0402-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)