Abstract
Live-cell imaging technique contributes to the progress of modern life science, especially in the field of neurobiology. Molecular mechanism of regulation in synapse formation, maturation, elimination, and remodeling can be effectively studied by imaging of living neurons, neural tissues isolated in vitro, and neural circuits functioning in vivo. In vitro imaging of synapse development by expression of fluorescent protein-tagged synaptic molecules in single neurons revealed rapid recruitment of synaptic molecules and structural differentiation that can be completed within several hours. In vivo two-photon excitation microscopy enabled direct measurement of synapse turnover in living animals. Although more than 90% of spine synapses are stabilized for more than months in the adult mouse neocortex, neural circuits were found to be under active rewiring in the early postnatal period, with the rate of synapse turnover comparable to that in dissociated cultures. Subsequent stabilization of spine synapse dynamics takes place at postnatal 3–4 weeks. Because multiple mouse models of autism spectrum disorders show identical phenotypes in upregulation of spine turnover at postnatal 3 weeks, prolonged functional impairment in the adult neocortex may be derived from increased misconnection of developing cortical neurons through accelerated turnover. Imaging of synapse turnover in vitro and in vivo is an indispensable experimental approach to understanding the construction principles of neural circuits in both physiological and pathological conditions.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
- Live-cell imaging
- Fluorescent proteins
- Synapse
- Postsynaptic density
- Two-photon microscopy
- In vivo imaging
- Autism
1 Synapse, Neuron, and Neural Network
The brain is a special organ dedicated for information processing that enables sensory perception, motor control and higher cognitive functions. Network of neurons is responsible for transmission, integration, and storage of information in the brain. Therefore, comprehensive description of complex neural network and extraction of the basic principles that govern the connectivity are essential in our understanding of brain function. This task is not simple and requires multiple-level analyses of the nervous system.
Neurons develop highly branched dendrites, which receive a large number of synaptic inputs, together with a long axon that enables connection with target neurons located in distant brain regions. Presynaptic axons contain synaptic vesicles and their exocytosis is coupled with arrival of action potentials (Munno and Syed 2003). Neurotransmitters released from presynaptic sites are immediately captured by postsynaptic receptors, which trigger either changes in postsynaptic membrane potential or activation of second messengers. Postsynaptic sites of neocortical pyramidal neurons form dendritic spines, which are tiny protrusions with a variety of morphology (Yuste and Bonhoeffer 2004). Neurotransmitter receptors on the cell surface are concentrated at postsynaptic densities (PSDs), a specialized region within dendritic spines (Okabe 2007). PSDs can be recognized as electron-dense structure by conventional transmission electron microscopy.
Connectivity of neurons is not homogeneous but distinct between different brain regions. In the neocortex, two types of neurons, excitatory neurons and inhibitory neurons, form local network. Excitatory pyramidal neurons release glutamate and inhibitory interneurons release γ-aminobutyric acid (GABA) from the axon terminal. The main information processing in the neocortex is mediated by connection between excitatory pyramidal neurons. Inhibitory interneurons regulate information processing by applying adequate suppression to the system. The most important question in the analyses of neocortical neuronal circuit is the relationship between neuronal connectivity and the mechanism of information processing. However, effective analytical methods that can achieve comprehensive and quantitative analyses of neural connectivity in the neocortex are not yet available at present.
On the level of the whole brain, reliable tracing technology of neuronal projections between distant brain regions is essential. This may be achieved by high resolution magnetic resonance imaging (MRI) in combination with injection of tracers and subsequent detection of labeled projections (Grandjean et al. 2017). Development of diffusion weighted imaging is rapid, but precise determination of axonal fibers is still difficult to achieve (Jbabdi et al. 2015). Physical tracers, such as fluorescent dyes and small molecules that can be taken up by living neurons, have been traditionally applied to neuron labeling, but these classical tracers cannot distinguish cell types, such as pyramidal neurons and interneurons, and also are not effective enough for complete detection of all axonal structures. Therefore, new genetic approaches for effective labeling of axons should be developed and applied for this purpose.
These arguments on the hierarchical architecture of the nervous system and the limitations of the available techniques indicate that it is important to develop new methodologies that integrate morphological and functional information on different scales. On the level of single synapses, high resolution imaging of synaptic structure and functional synaptic molecules are essential tools in modern neurobiology and in vitro imaging studies provided rich information about molecular mechanisms of synapse formation, maturation, elimination and remodeling. On the level of local neuronal circuits, morphological dynamics of axons, dendrites, and synapses in vivo can be recorded by two-photon excitation laser scanning microscopy in combination with appropriate surgical techniques for chronic imaging. In the following chapters, we will discuss these two major microscopic approaches to synapse dynamics.
2 In Vitro Imaging of Dynamic Synapses
In the initial attempt of understanding synapse development, researchers performed staining of neurons in culture or in fixed brain sections using antibodies specific for synaptic molecules and collected static images at different time points (Cohen-Cory 2002). In many cases, these studies utilized either the neocortex or the hippocampus as model systems and reported gradual increases in both presynaptic and postsynaptic immunoreactivity along the time course of development (Fletcher et al. 1991). The electron microscopic observation also supported the data of immunohistochemistry (Harris et al. 1992). These morphological data were taken as evidence for the slow differentiation process of synapses after their formation and the concept of turnover of single synapses had not been introduced.
This situation was changed dramatically after introduction of the single synapse imaging technique applied to living neurons in dissociated culture system (Okabe 2017). Researchers initially predicted that nascent synapses are formed between neurons only in the early period of culture and these immature synapses gradually reach the mature stage in the following 2–3 weeks of the culture period. However, this prediction was not correct and live-cell imaging revealed continual formation of nascent synapses throughout the period of neuronal culture. For example, when a prominent PSD scaffold protein, PSD-95, tagged with GFP (PSD-95-GFP) is expressed in cultured hippocampal neurons, PSD-95-GFP is nicely localized to the PSDs within spines (Okabe et al. 1999). Distribution of PSD-95 puncta imaged at two time points with intervals of 24 h is markedly different, indicating that formation of individual synapses is a rapid process that takes place within a day. Quantitative analysis indicated that about 20% of synapses turnover within 24 h even when cultured neurons have been maintained for more than 3 weeks. More detailed analysis of both presynaptic molecular markers and postsynaptic spine structure further supported the idea of continual formation of nascent synapses in culture (Okabe et al. 2001). Namely, when new PSD-95 puncta appear, there is concomitant formation of presynaptic synaptophysin puncta and postsynaptic spine formation. These three processes are synchronized and complete within several hours, strongly suggesting that rapid establishment of the complete synaptic structure and molecular assembly is a highly frequent event throughout the development of neural circuits in culture system.
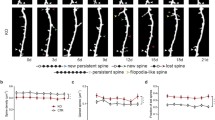
It should also be emphasized that synapse elimination is another important component of the synapse dynamics that takes place throughout the culture period (Fig. 19.1). Elimination rate of synapses is high in the mature neurons that have been maintained for more than 3 weeks in culture. Importantly, synapse formation and elimination are balanced and the subtraction of synapse elimination from formation is a good predictor for the overall trend of synapse density increase. Long-term observation of the synapse population on the same dendrites is possible by culturing neurons isolated from transgenic mouse lines expressing PSD-95-GFP or another abundant postsynaptic scaffolding protein, Homer1c, tagged with GFP (Ebihara et al. 2003). When the fate of single PSD structures is monitored over days, elimination of newly formed synapses and concomitant appearance of new synapses in the vicinity of lost synapses are frequently observed. This experiment confirmed the importance of the balance between synapse formation and elimination in setting the density of synapses within the optimal range.
Formation and subsequent stabilization of synapses. Synapse formation starts from appearance of nascent synaptic structure with a small PSD and a few synaptic vesicles. Subsequent increase in PSD size and accumulation of actin filaments in spines stabilizes postsynaptic structure. Recruitment of more synaptic vesicles in the presynaptic bouton and insertion of glutamate receptors are important in initiation of synaptic transmission. Not all of the newly generated synapses go through the entire process of synapse maturation in the developmental period. A large number of newly generated synapses reverse the direction and are lost after reduction of their structure
How synapse density is regulated to be within the optimal range? To answer this question, candidate molecular pathways involved in the setting of spine density after maturation of the neural circuit should be identified. Our recent study of calcium-calmodulin-dependent protein kinase II-alpha (CaMKIIα) activity in regulation of spine density indicated that pyramidal neurons without CaMKIIα activity upregulate the rate of spine addition and show increase in spine density (Cornelia Koeberle et al. 2017). This CaMKIIα-dependent regulation of spine density is through the pathway of synaptic Ras GTPase-activating protein (synGAP) and the activity of the small GTPase Rap1. Because CaMKIIα protein content and its activity increase prominently in the postnatal period, this upregulation enhances Rap1 activity, which negatively regulates spine stability via increase in actin dynamics.
It is possible that the balance between formation and elimination of synapses is regulated not only by postsynaptic mechanisms, such as CaMKII activity, but also by the mechanisms involving presynaptic activity. Indeed, we identified presynaptic release of bone morphogenetic protein 4 (BMP4), a well-known signaling molecule for neuroepithelial differentiation in the early embryo, as a major regulator of synapse elimination in neural circuits of the hippocampus and the neocortex (Higashi et al. 2018). BMP4 is transported in dense-core vesicles along the axon, released locally in the vicinity of synapses in an activity-dependent manner, tethered to the plasma membrane of the perisynaptic region, and destabilizes nearby synapses. This effect is mediated through BMP receptors on the axonal surface and subsequent activation of canonical Smad pathway. This study clearly shows the existence of specific molecular pathway that controls synapse elimination with a spatial precision of single synapses.
3 In Vivo Imaging of Dynamic Synapses
Introduction of the technique of two-photon excitation laser scanning microscopy opened the way toward in vivo long-term monitoring of single synapses. In combination with appropriate surgical techniques to create optical windows over the brain parenchyma, single synapses can be followed for hours, days, and months in living animals (Grutzendler et al. 2002; Trachtenberg et al. 2002). Previous data collected in reduced preparations, such as dissociated neuron culture and slice preparations, can now be confirmed in the intact neuronal circuits in vivo. As in the case of synapse detection in culture, combination of structural markers, such as dendritic spines, and molecular markers, such as PSD-95-GFP, can increase the reliability of detecting spine synapses formed onto neocortical pyramidal neurons (Gray et al. 2006). One drawback in the approach of in vivo two-photon imaging is that only limited brain areas can be accessed by this technique, mainly because penetration of infrared light is limited to the cortical layer of less than 1000 μm in depth from the surface (Helmchen and Denk 2005). By using endoscope technology, synapse turnover in the hippocampus and other subcortical areas can be measured, but the technique is less reliable than the two-photon imaging, mainly due to the lower resolution (Attardo et al. 2015).
As stated previously, comparison of tissue sections with immunohistochemical staining of synaptic molecules revealed progressive increase in synaptic molecules in the early postnatal cortex. This increase in synaptic molecules is associated with increase in the density of synaptic structures detected by electron microscopy. These studies collectively indicate a stereotyped pattern of neural circuit development. On the other hand, synapse imaging studies in vitro revealed highly dynamic nature of individual synapses and persistent exchange of synapses even in the mature stage of neurons in culture. Therefore, one of the major questions is whether synapse turnover persists in the adult neocortex or hippocampus. The current agreement to this question is that, at least in the mouse neocortex, more than 90% of synapses are highly stabilized and can be maintained for several months (Zuo et al. 2005; Holtmaat et al. 2009). This observation indicates that synapse maturation in dissociated neurons does not reach the level of mature neocortex in vivo. This difference may be related to many factors that are not present in the culture environment, such as glial cells, neurovascular coupling, and synaptic connections with remote brain regions. Interestingly, recent in vivo imaging by the endoscopic technique reported continual remodeling of a large proportion of synapses in the mature hippocampus, suggesting regional heterogeneity in synapse stability (Attardo et al. 2015).
In vivo two-photon imaging can also be applied to developing neural circuits in the early postnatal period. However, it is not an easy task to create imaging windows without inducing activation of glial cells in young animals. The cranial bone is thin and soft and the animals are less resistant to inappropriate surgery and anesthesia. Our group successfully applied the technique of thinning the skull to the thickness appropriate for in vivo imaging at postnatal 2 and 3 weeks of mice and obtained the reliable data of spine turnover in the multiple neocortical areas (Isshiki et al. 2014). The data showed extensive turnover of spines positive with PSD-95-GFP and the turnover rate (up to 15–20% over 24 h) was comparable to that measured in dissociated neurons in culture. Importantly, the basic principle that the balance between formation and elimination of spines determines the net change in total spine density holds true in the case of in vivo synapse turnover. At postnatal 2 weeks, spine formation largely exceeds spine elimination, supporting rapid increase in the spine density. In turn, the two components are both suppressed and are balanced at postnatal 4 weeks, when the synapse density reaches the peak and starts to decline gradually afterwards.
Interestingly, this developmental profile of synapse density is common across different species including human, with different time scales. Previous studies show that the peak of spine density in the human neocortex is at the age of 1–4 years (Fig. 19.2) (Huttenlocher and Dabholkar 1997). The rate of spine increase is high before reaching the peak and the slow decline of spine density follows and persists thereafter. This specific temporal profile is preserved in both monkey (Elston et al. 2009) and mouse neocortex (Aceti et al. 2015). Synapse imaging data both in vitro and in vivo suggest that the initial phase of rapid construction of synaptic connectivity is regulated by the dynamic balance between synapse formation and elimination. Also this period corresponds to differentiation of specific functions in individual neocortical areas, such as sensory, motor, and association cortices, in response to the extrinsic signals from the environment. It is reasonable to assume that the intricate balance of synapse turnover in this early phase of circuit development profoundly affect the performance of the neocortical circuits thereafter.
4 In Vivo Imaging of Neocortical Circuits in Mouse Models of Developmental Disorders
Impairment in cortical information processing is thought to underlie behavioral deficits in autism spectrum disorders (ASDs). Advancement in the diagnostic techniques enabled screening of ASDs by 3 years of age. Because of relatively small changes in the cellular architecture and little signs of neurodegeneration in the patient brain, pathogenesis of ASDs is proposed to be associated with formation of inappropriate connectivity between cortical neurons (Bourgeron 2015). In support of this hypothesis, recent genetic studies of copy number variants (CNVs) and gene mutations reported that genes encoding synaptic cell adhesion molecules (postsynaptic cell adhesion molecules neurogilin-3 and -4, and their presynaptic binding partners neurexin-1), and PSD scaffolding molecules (Shank2 and Shank3) are involved in increasing the risk of ASDs. These genetic studies indicate that detection of proper synaptic binding partners and maintenance of postsynaptic structures are impaired by these genetic mutations and subsequent accumulation of mismatches in neuronal connectivity may lead to dysfunction of local neuronal circuits in the neocortex. Once the neocortical neuronal connectivity is stabilized in the mature brain, the impairment in the circuit becomes resistant to therapeutic intervention and the associated clinical symptoms, such as repetitive behavior and reduced social communication, may persist throughout life.
Recent progress in mouse molecular genetics enable us to create mouse models of ASDs. Several types of CNVs have been reported in ASD patients and the most frequently reported copy number variation in ASD, the duplication of 15q11-13, has been mimicked in mice by creating the genomic duplication in the corresponding genomic region of the mouse chromosome 7 (patDp/+ mice)(Nakatani et al. 2009). This mouse model shows impairment in social behaviors and imbalance of excitatory and inhibitory synaptic transmission, which are common phenotypes seen in mouse models of ASDs. Another reliable model of ASD was created by introducing a point mutation in the coding region of neuroligin 3 (NLG R451C mice) (Tabuchi et al. 2007). This point mutation is responsible for the alterations again in the balance of excitatory and inhibitory synapses in the neocortex, together with impairment in synaptic plasticity and social behaviors. These two models are heterogeneous in their genetic properties, but show similar impairments in the functions of neocortical circuits and behaviors. Therefore, it is reasonable to expect common alterations in neocortical synapse development in the early postnatal period. Initial quantitative analysis of spine and synapse, together with the analysis of their morphology, could not detect any significant change in these mutant mice, suggesting that the static analysis of spines and synapses is not sufficient to detect the phenotypes (Isshiki et al. 2014). In spite of little changes in spine synapse density and morphology, in vivo two-photon imaging of spine synapses in the neocortex of patDp/+ mice and NLG R451C mice revealed prominent upregulation of spine turnover at postnatal 3 weeks. This upregulation is more prominent in spines containing PSD-95 clusters, suggesting that spines with functional synaptic connectivity are more severely affected. In addition, the phenotype is specific to spines receiving intracortical projections while spines contacting with the thalamocortical projections are unaffected. This selective impairment in the intracortical connections may be responsible for behavioral phenotypes of the mutant mice, such as deficits in social behavior, which are thought to be related to functions in the association cortex. We observed similar upregulation of cortical spine synapses in the third ASD mouse model, BTBR mice. This strain also exhibits clear impairment in ASD-related behaviors, such as social interactions and repetitive behaviors (McFarlane et al. 2008). In Fmr1 knockout mice, a model of fragile X syndrome and syndromic autism, spine turnover in the neocortex is also reported to be upregulated (Pan et al. 2010). Taken together, these results indicate that higher synapse turnover in the early postnatal period is a shared synapse-level phenotype across ASD mouse models.
Based on our observation of synapse dynamics in multiple mouse models of ASDs, together with reports from other laboratories, we propose that multiple genetic and environmental risk factors operate in the early postnatal period and their effects converge towards upregulation of cortical synapse turnover, which subsequently induce increased misconnections of cortical neurons and its dysfunction (Fig. 19.3). Although it is widely accepted that the imbalance between excitatory and inhibitory synapses underlies the dysfunction of ASD brain, the relationship between enhanced synapse turnover and the excitatory-inhibitory imbalance has not yet been clarified (Han et al. 2012; Nakai et al. 2017). One possible explanation is that the late maturation of inhibitory neurons in the cortex requires an adequate level of excitatory inputs within the cortex, and this excitatory drive is reduced after initial increase in the misconnections caused by increased synapse turnover. This model is consistent with our observation of reduced activity in layer II/III neurons after enhancement of whisker-related sensory experience in both patDp/+ mice and NLG R451C mice. If the activity in the neocortex is reduced, inhibitory system may be less developed, and after maturation of the system, the local cortical circuits will become less resistant to the excess amount of sensory inputs. This scenario can explain higher probability of epileptic episodes in multiple mouse models of ASDs and also in ASD patients.
A proposed model of neural circuit dysfunction in mouse models of ASD. Synaptic connections increase during the early postnatal period. Excess in synapse formation and elimination seen in the mouse models of ASD increases the probability of synaptic misconnection. This inhibits proper activation of neural circuits in the neocortex in the mature brain and leads to behavioral phenotypes
5 Perspectives
Progress in synapse neurobiology has been accelerated by development of novel imaging technologies. Fluorescent protein-based live cell imaging is a powerful approach toward detection of single synapses and their remodeling in culture. Application of two-photon excitation microscopy to visualization of synapse dynamics in the brain of living mice provided indispensable information about stability and lifetime of individual spine synapses. This technique was also proved to be useful in detection of synapse pathology in mice containing genetic mutations seen in human patients of neurodevelopmental disorders. In future, further advancements in both light microscopic and electron microscopic techniques of synapse visualization will promote our understanding of neural circuit construction and remodeling in development, maturation, and aging.
References
Aceti M, Creson TK, Vaissiere T et al (2015) Syngap1 haploinsufficiency damages a postnatal critical period of pyramidal cell structural maturation linked to cortical circuit assembly. Biol Psychiatry 77:805–815. https://doi.org/10.1016/j.biopsych.2014.08.001
Attardo A, Fitzgerald JE, Schnitzer MJ (2015) Impermanence of dendritic spines in live adult CA1 hippocampus. Nature 523:592–596. https://doi.org/10.1038/nature14467
Bourgeron T (2015) From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat Rev Neurosci 16:551–563. https://doi.org/10.1038/nrn3992
Cohen-Cory S (2002) The developing synapse: construction and modulation of synaptic structures and circuits. Science 298:770–776. https://doi.org/10.1126/science.1075510
Cornelia Koeberle S, Tanaka S, Kuriu T et al (2017) Developmental stage-dependent regulation of spine formation by calcium-calmodulin-dependent protein kinase IIα and Rap1. Sci Rep 7. https://doi.org/10.1038/s41598-017-13728-y
Ebihara T, Kawabata I, Usui S et al (2003) Synchronized formation and remodeling of postsynaptic densities: long-term visualization of hippocampal neurons expressing postsynaptic density proteins tagged with green fluorescent protein. J Neurosci 23:2170–2181. 23/6/2170 [pii]
Elston GN, Oga T, Fujita I (2009) Spinogenesis and pruning scales across functional hierarchies. J Neurosci 29:3271–3275. https://doi.org/10.1523/JNEUROSCI.5216-08.2009
Fletcher TL, Cameron P, De Camilli P, Banker G (1991) The distribution of synapsin I and synaptophysin in hippocampal neurons developing in culture. J Neurosci 11:1617–1626. https://doi.org/10.1103/PhysRevLett.114.173002
Grandjean J, Zerbi V, Balsters J et al (2017) The structural basis of large-scale functional connectivity in the mouse. J Neurosci:438–417. https://doi.org/10.1523/JNEUROSCI.0438-17.2017
Gray NW, Weimer RM, Bureau I, Svoboda K (2006) Rapid redistribution of synaptic PSD-95 in the neocortex in vivo. PLoS Biol 4:2065–2075. https://doi.org/10.1371/journal.pbio.0040370
Grutzendler J, Kasthuri N, Gan WBW-B (2002) Long-term dendritic spine stability in the adult cortex. Nature 420:812–816. https://doi.org/10.1038/nature01151.1
Han S, Tai C, Westenbroek RE et al (2012) Autistic-like behaviour in Scn1a +− mice and rescue by enhanced GABA-mediated neurotransmission. Nature 489:385–390. https://doi.org/10.1038/nature11356
Harris KM, Jensen FE, Tsao B (1992) Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci 12:2685–2705. https://doi.org/10.1016/j.tcb.2009.06.001
Helmchen F, Denk W (2005) Deep tissue two-photon microscopy. Nat Methods 2:932–940. https://doi.org/10.1038/nmeth818
Higashi T, Tanaka S, IIda T, Okabe S (2018) Synapse elimination triggered by BMP4 exocytosis and presynaptic BMP receptor activation. Cell Rep 22(4):919–929
Holtmaat A, Bonhoeffer T, Chow DK et al (2009) Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat Protoc 4:1128–1144. https://doi.org/10.1038/nprot.2009.89.Long-term
Huttenlocher PR, Dabholkar AS (1997) Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 387:167–178. https://doi.org/10.1002/(SICI)1096-9861(19971020)387:2<167::AID-CNE1>3.0.CO;2-Z
Isshiki M, Tanaka S, Kuriu T et al (2014) Enhanced synapse remodelling as a common phenotype in mouse models of autism. Nat Commun 5:4742. https://doi.org/10.1038/ncomms5742
Jbabdi S, Sotiropoulos SN, Haber SN et al (2015) Measuring macroscopic brain connections in vivo. Nat Neurosci 18:1546–1555. https://doi.org/10.1038/nn.4134
McFarlane HG, Kusek GK, Yang M et al (2008) Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav 7:152–163. https://doi.org/10.1111/j.1601-183X.2007.00330.x
Munno DW, Syed NI (2003) Synaptogenesis in the CNS: an odyssey from wiring together to firing together. J Physiol 552:1–11. https://doi.org/10.1113/jphysiol.2003.045062
Nakai N, Nagano M, Saitow F et al (2017) Serotonin rebalances cortical tuning and behavior linked to autism symptoms in 15q11-13 CNV mice. Sci Adv 3:e1603001. https://doi.org/10.1126/sciadv.1603001
Nakatani J, Tamada K, Hatanaka F et al (2009) Abnormal behavior in a chromosome- engineered mouse model for human 15q11-13 duplication seen in autism. Cell 137:1235–1246. https://doi.org/10.1016/j.cell.2009.04.024
Okabe S (2007) Molecular anatomy of the postsynaptic density. Mol Cell Neurosci 34:503–518. https://doi.org/10.1016/j.mcn.2007.01.006
Okabe S (2017) Fluorescence imaging of synapse dynamics in normal circuit maturation and in developmental disorders. Proc Jpn Acad Ser B Phys Biol Sci 93:483–497. https://doi.org/10.2183/pjab.93.029
Okabe S, Kim HD, Miwa A et al (1999) Continual remodeling of postsynaptic density and its regulation by synaptic activity. Nat Neurosci 2:804–811. https://doi.org/10.1038/12175
Okabe S, Miwa A, Okado H (2001) Spine formation and correlated assembly of presynaptic and postsynaptic molecules. J Neurosci 21:6105–6114. 21/16/6105 [pii]
Pan F, Aldridge GM, Greenough WT, Gan W-B (2010) Dendritic spine instability and insensitivity to modulation by sensory experience in a mouse model of fragile X syndrome. Proc Natl Acad Sci U S A 107:17768–17773. https://doi.org/10.1073/pnas.1012496107
Tabuchi K, Blundell J, Etherton MR et al (2007) A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science 318:71–76. https://doi.org/10.1126/science.1146221
Trachtenberg JT, Chen BE, Knott GW et al (2002) Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 420:788–794. https://doi.org/10.1038/nature01273
Yuste R, Bonhoeffer T (2004) Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat Rev Neurosci 5:24–34. https://doi.org/10.1038/nrn1300
Zuo Y, Lin A, Chang P, Gan WB (2005) Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron 46:181–189. https://doi.org/10.1016/j.neuron.2005.04.001
Acknowledgements
This study was supported by the Uehara Memorial Foundation, Grants-in-Aid for Scientific Research (17H01387, 26250014 and 25117006), and Core Research for Evolutional Science and Technology from the Japanese Science and Technology Agency (JPMJCR14W2).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
1 Supplementary Electronic Material (S)
(MP4 543056 kb)
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2020 The Author(s)
About this paper
Cite this paper
Okabe, S. (2020). Imaging Synapse Formation and Remodeling In Vitro and In Vivo. In: Toyama, Y., Miyawaki, A., Nakamura, M., Jinzaki, M. (eds) Make Life Visible. Springer, Singapore. https://doi.org/10.1007/978-981-13-7908-6_19
Download citation
DOI: https://doi.org/10.1007/978-981-13-7908-6_19
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-7907-9
Online ISBN: 978-981-13-7908-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)