Abstract
Filamentous fungi are widely used for production of cellulase and other cellulolytic enzymes. Metabolic engineering of filamentous fungal strains has been applied to improve enzyme production, and rapid progress has been made in the recent years. In this chapter, genetic tools and methods to develop superior enzyme producers are summarized, which includes establishment of genetic modification systems, selection and redesign of promoters, and metabolic engineering using either native transcription factors or artificial ones. In addition, enhancement of cellulase production through morphology engineering was also discussed. Emerging tools including CRISPR-Cas9-based genome editing and synthetic biology are highlighted, which are speeding up mechanisms elucidation and strain development, and will further facilitate economic cellulolytic enzyme production.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Filamentous fungi
- Trichoderma reesei
- Cellulase
- Promoter engineering
- Artificial transcription factor
- Genome editing
- Metabolic engineering
1 Introduction
Lignocellulosic biomass are abundant in nature, which includes agricultural residues, forestry wastes, as well as energy crops. In China, it was estimated that 600–700 million tons of agricultural residues are produced annually (Xie et al. 2010). Production of biofuels, especially cellulosic ethanol (Zhao et al. 2016), as well as biochemicals from these cellulosic biomass (Zhang et al. 2016a) has received considerable interest in the past decades. To release fermentable sugars from lignocellulosic biomass, enzymatic hydrolysis is commonly used. Therefore, efficient production of cellulolytic enzymes is important for the process economy.
Filamentous fungi are major cellulase producers and are also widely utilized for producing other industrial enzymes. Cellulase production from the fungal species of Trichoderma reesei (Bischof et al. 2016), Neurospora crassa (Znameroski et al. 2012), Aspergillus niger (Stricker et al. 2008), and Penicillium oxalicum (Li et al. 2017b) is of special interests and have been intensively studied. In recent years, filamentous fungi have gained attention as cell factories for enzyme production. However, high cost of cellulase production is still one of the bottlenecks for industrialization of lignocellulosic bioconversion.
More than 70 years ago, the ascomycete T. reesei was isolated, and its potential to encode cellulase and hemicellulase for biomass degradation was investigated (Bischof et al. 2016). Despite the fact that some other filamentous fungi can produce efficient cellulose-hydrolyzing enzyme cocktail, T. reesei is still widely used for industrial cellulase production. Besides, genetic engineering of T. reesei has been conducted for more than 30 years, which benefits regulatory mechanisms studies on enzyme production and have achieved powerful cellulolytic mixtures by the fungus (Peterson and Nevalainen 2012). Therefore, in this chapter, we would mainly focus on genetic engineering of T. reesei for cellulase production.
In the past decades, the regulatory network of cellulase expression and enzyme secretion has been investigated in details. Metabolic engineering of the widely used cellulase producer T. reesei has been reviewed previously (Kubicek et al. 2009). In the recent years, development of high-throughput sequencing technologies has led to remarkable advancement in systems biology studies, and abundant data have been obtained including genomic sequences, global transcriptomic profiles from RNA sequencing, proteomics data and so on (Liu et al. 2013). On the other hand, new methods and tools have been developed to improve the efficiency of genetic engineering of filamentous fungi. In this context, it is optimistic to see the new era of strain development, which is based on deeper understanding of the regulatory mechanisms of enzyme production and advanced technologies of genetic engineering.
In this chapter, we summarized recent progress of genetic tools and methods to develop superior enzyme producers, which includes establishment of genetic modification systems, selection and redesign of promoters, and metabolic engineering using either native transcription factors or artificial ones. Enhancement of cellulase production through morphology engineering was also discussed. Emerging tools including genome editing and synthetic biology are highlighted, which are speeding up studies on mechanism elucidation and strain development, and will enable economic cellulase production for bioconversion of lignocellulosic biomass.
2 Selective Markers for Genetic Engineering of Filamentous Fungi
Establishment of transformation methods is the first step for genetic engineering of filamentous fungi. The transformation methods mainly include protoplast transformation, electroporation, and Agrobacterium-mediated transformation. The detailed transformation methods were described elsewhere (Malmierca et al. 2015; Chakraborty 2015; Frandsen 2015). Besides the transformation methods, efficient selective markers used in filamentous fungi are also key factors for obtaining the target transformants. Many dominant selectable markers including the nutritional markers and positive selection resistance markers are being employed, which were summarized in Table 2.1.
Nutritional markers of pyrG gene (A. nidulans) or pyr4 gene (N. crassa) encoding orotidine-5′-phosphate decarboxylase were used to complement uracil auxotrophs (Gruber et al. 1990; Gao et al. 2012). pyrG/pyr4 deletion mutants were easily selected by 5-fluoroorotic acid (5-FOA) resistance, whereas the wild-type strains could not grow on the toxic compound 5-FOA. Similar systems employing the trpC (Yelton et al. 1984) or acuD (Beri and Turner 1987) genes as the auxotrophic markers are also available. In addition, the niaD marker encoding the nitrate reductase was widely used to obtain the chlorate-resistant mutant, which has been successfully used in transformation of A. niger (Unkles et al. 1989). Moreover, the amdS gene (encoding acetamidase) was also employed as the nutritional markers for Aspergillus (Kelly and Hynes 1985) and thermophilic Myceliophthora (Liu et al. 2017) species by selecting for acetamide utilization, since acetamide is a poor nitrogen source for the wild-type strains.
The use of nutritional markers is important in the progress of genetic engineering of filamentous fungi. However, auxotrophic selection requires strains with specific mutations and culturing in specialized media, which may have negative effects on cell growth. In contrast, the positive resistance markers are nowadays widely applied in the studies of filamentous fungi transformation with the advantage of that the genotype of the recipient strains need not to be known in advance (Ruiz-Díez 2002). The transformation method with the resistance markers is easy to operate, and vectors carrying the resistance marker gene cassette can be transformed into the recipient strains directly, and the mutants tolerate no background growth in the standard media. The hygromycin-B resistant marker was used in the early study of A. nidulans transformation (Punt et al. 1987) and T. reesei (Zhang et al. 2017; Li et al. 2017a). The other frequently used selection resistance marker genes include pyrithiamine gene (ptrA) (Wang et al. 2013a), phleomycin gene (Chen et al. 2016), and phosphinothricin gene (Ahuja and Punekar 2008). However, the major drawback of using these kinds of resistance markers is that the resistance of the wild-type strains may not show significant dominance, resulting in selection difficulties. Moreover, some of those antibiotics are often expensive.
Although the transformation methods and useful resistance markers have been established currently, the lack of selective markers and the problem of false-positive transformants are still the obstacles of filamentous fungi genetic engineering. Screening the positive transformants with two selection markers, for example, with fluorescent protein and hygromycin-B as double reporter genes, would be helpful (Noh et al. 2010).
In cellulolytic synthesis filamentous fungi, such as Trichoderma sp. and Penicillium sp., no stable autonomously replicating plasmid was found so far. Therefore, once the resistance marker was introduced, it would be integrated into the host genomic DNA, which was difficult to remove for a second round of genetic engineering. The Cre-loxP system has been developed as an important molecular tool to overcome these genetic limitations and also should reduce public concerns involving environmental release of resistant strains. The Cre recombinase from bacteriophage P1 of Escherichia coli catalyzes recombination between the two 34 bp loxP sites, each of them has two 13 bp Cre-binding sites which are interrupted by an 8 bp spacer region (Sternberg and Hamilton 1981). The system was first investigated in E. coli (Sternberg and Hamilton 1981) and utilized widely later in fungi, such as T. reesei (Steiger et al. 2011) and Aspergillus sp. (Forment et al. 2006). The expression of Cre recombinase was usually controlled by inducible promoters thus providing a means to switch on or off. In filamentous fungi, the xylanase promoter was frequently employed which can be induced by many kinds of carbohydrates, such as xylose and cellulose (Steiger et al. 2011; Forment et al. 2006). Moreover, Tet-on and Cre-loxP-based systems were successfully employed in P. oxalicum and Cre recombinase was activated by doxycycline which was controlled by the Tet-on system (Jiang et al. 2016).
The FLP/FRT recombination system was an alternative to the Cre-loxP system. Similar with the Cre-loxP system, heterologous expression of FLP recombinase from 2µm plasmid of Saccharomyces cerevisiae could facilitate resistance marker gene disruption between the two specific DNA sequences in a single cell. The system has been successfully tested and optimized in fungi P. chrysogenum (Kopke et al. 2010). The resulting marker genes deletion strain can be used as a recipient strain for further genetic engineering reapplying the same system.
3 Promoter Engineering to Improve Cellulase Production
T. reesei can secrete many types of cellulolytic enzymes, of which cellubiohydrolase 1 (CBH1) is the major component of all secreted proteins. Therefore, cbh1 promoter has been widely used to induce hyper production of target proteins in T. reesei. It is well-known that β-glucosidase (BGL) activity in T. reesei is very low. Therefore, overexpression of BGL and other cellulase components in T. reesei using the strong inducible promoter of cbh1 and cbh2 have been reported, which can significantly improve the corresponding gene expression and ultimately can significantly improve the overall hydrolase activities (Ma et al. 2011; Li et al. 2017a). Improved enzyme production was also reported using the artificial four-copy cbh1 promoter with repeated positive transcriptional elements and deletion of possible glucose repressor-binding sites (Zhang et al. 2010). Through the rational design of the promoter, it will also significantly affect the transcription and expression of the corresponding genes, such as the engineered cbh1 promoter, in which the cellulase negative regulatory factor Cre1 binding sites were replaced by transcriptional activator Ace2 and Hap2/3/5 complex binding sites, and the engineered cbh 1 promoter intensity was increased by 5.5 and 7.4 times under induced and inhibited conditions, respectively (Zou et al. 2012).
Wang et al. developed a promoter collection for the expression of alkaline cellulase genes in T. reesei, which was employed to construct different expression systems based on several promoters and terminators of T. reesei, and produced the most efficient enzyme expression system for bio-stoning, which relied on the action of two enzymes synergistically working to modify the fabric surface (Wang et al. 2014). Therefore, it is necessary to dig out more strong promoters through the transcriptomic data to increase the expression level of the target glycoside hydrolase gene without affecting the main metabolic pathways and the expression of main glycoside hydrolases.
Despite some progress presented above, promoters of filamentous fungi have been only limitedly studied. For advanced metabolic engineering and synthetic biology studies of these important fungal species, artificial promoters such as hybrid promoters, as well as synthetic minimal promoters, which have been successful in budding yeast strains of S. cerevisiae (Blazeck et al. 2012; Redden and Alper 2015), can be explored, which will provide different expression levels of key enzymes and regulators for strain development. Construction of modular vectors containing synthetic promoters is the basis for synthetic biology manipulation of filamentous fungi (reviewed by Gupta et al. 2016), and design or screening promoter library can also be combined with different regulators which will be described in the following section to generate functional diversities.
4 Engineering of Transcription Regulators to Improve Cellulase Production
It is well known that cellulase production is regulated on transcription level, and abundant transcription regulators have been identified in different filamentous fungi (Benocci et al. 2017). Comparative transcriptome analysis showed that different types of glycoside hydrolases were induced under different induction conditions. For example, the components of extracellular secretion proteins are different significantly for T. reesei when the cellulases were induced by the pretreated lignocellulose, xylan, and sophorose (Hakkinen et al. 2012). In addition, environmental factors, such as pH (Li et al. 2013) and light (Schmoll et al. 2012), also affect cellulase induction. Therefore, understanding the regulatory network is important to engineer cellulase production in filamentous fungi.
Regulation of biomass-degrading enzymes in T. reesei was recently reviewed (Gupta et al. 2016), and new findings are also emerging in the later studies. The cellulolytic and xylanolytic gene expression in T. reesei is coordinately regulated by the action of at least four transcriptional activators (Xyr1, Ace2, Ace3, and Hap2/3/5 complex) and three repressors (Cre1, Ace1, and the recently reported regulator Rce1) (reviewed by Seiboth et al. 2012; Cao et al. 2017). Xyr1 (xylanase regulator 1) is the key positive transcriptional activator; and lack of xyr1 eliminates the induction of cellulase and xylanase by all known inducers (Stricker et al. 2006). Cre1 is the major negative regulator mediating carbon catabolite repression (CCR) by inhibiting both basal and the inducible expressions, and moreover also prevents xyr1 gene expression. In T. reesei, cre1 transcription was autoregulated under non-inducing conditions, and xyr1 gene was therefore shown at a low basal level (Lichius et al. 2014). Overexpression of xyr1 improved glycoside hydrolase production under inducing conditions, while deletion of cre1 eliminated CCR, but the catabolic de-repression was not sufficient to increase glycoside hydrolase production, suggesting that hyperproduction is still inducer dependent (Wang et al. 2013b; Nakari-Setala et al. 2009).
RNA interference was used to regulate the expression of Cre1 in T. koningii, causing the transcription of cre1 gene to be disturbed to varying degrees, and finally increased the total cellulase activity by more than two times (Wang et al. 2013b). In addition, Wang et al. also carried out overexpression of positive regulator Xyr1 in T. reesei and RNA interference of Ace1 at the same time. Under the double effect, extracellular protein secretion and filter paper activity from T. reesei Rut-C30 mutant are improved more than one time (Wang et al. 2013c).
Transcription factor proteins are consisted of DNA-binding domain and effector domain. Previous study showed that the chimeric transcription activator, which contains two DNA-binding domains from repressors Cre1 and Ace1 and effector domain from activator Ace2, could bind to both Cre1 and Ace1 binding sites in promoters of cellulase genes to activate gene expression (Su et al. 2009). Additionally, lots of xyr1 and cre1 binding sites exist in the promoter of major cellulase and xylanase gene (Castro et al. 2014). In T. reesei Rut-C30, Cre1 is truncated and loses its DNA binding ability, making the binding sites in the promoter idled (Seidl et al. 2008). In a recent report from our group, constitutively expression of an artificial activator containing both the Cre1 DNA-binding domain, the Xyr1 DNA-binding domain and effector domain, enhanced cellulase production in the presence of glucose (Zhang et al. 2017).
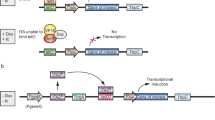
In addition to manipulating natural transcription factors, artificial transcription factor was also attempted to improve cellulase production in T. reesei. In the recent studies in our group, the designed artificial zinc finger transcription factor containing various DNA-binding domain and Gal4 effector domain was introduced into T. reesei Rut-C30, and cellulase hyper producer was screened. One of the mutants showed a 55% rise in filter paper activity and an 8.1-fold increased β-glucosidase activity (Zhang et al. 2016c). The scheme of the strain development using the artificial zinc finger protein was elucidated in Fig. 2.1. Interestingly, we found elevation of Trvib-1 in the selected mutant, and we tested further the effect of its overexpression on cellulase production in T. reesei Rut-C30. Vib-1 was reported to exert control of cellulase production in N. crassa (Xiong et al. 2014), and it was found that Trvib-1, the homologous gene of vib-1 from T. reesei, can complement the deletion of vib-1 in N. crassa. We found improvement of cellulase production in T. reesei Rut-C30 by overexpression of Trvib-1 (Zhang et al. 2018), and the involvement of this gene in regulation of cellulase production was also reported by another group (Ivanova et al. 2017). Therefore, novel regulatory mechanisms can be revealed by studying the mutant carrying the artificial transcription factor. It can also be expected that in the future, more artificial transcription factors can be used to modulate cellulase production in filamentous fungi.
Flow diagram for cellulase production by T. reesei Rut-C30 derivative strains engineered by an artificial transcription factor (ATF) library. Detailed information was described in the main text in Sect. 4
It is worth noting that the regulation of cellulase production is not only restricted at the transcription level but also affected by chromatin remodeling (Mello-de-Sousa et al. 2015). Studies on epigenetic control of cellulase production will offer novel metabolic engineering strategies to develop superior fungal strains.
5 Improvement of Cellulase Production by Morphology Engineering
Filamentous fungi exhibit complex morphological changes during submerged culture, which is of great interest for process optimization. Freely suspended mycelia and pellets with varying sizes can be found commonly in different filamentous fungal cultures. Although there are still some debates on the relationship of morphology and enzyme productivity, positive correlation of morphology with enzyme production was reported in T. reesei (Novy et al. 2016; Yu et al. 2012), N. crassa (Sun et al. 2014), and A. niger (Driouch et al. 2011). For example, relatively higher agitation speed increased pellet diameter and cellulase productivity of T. reesei Rut-C30, and on the other hand, cellulase activity and cell viability were sensitive to impeller shear (Yu et al. 2012). It is therefore important to control balanced pellet morphology, which is influenced by many process parameters, including medium composition, inoculation size, culture mode, pH value, temperature, osmolality, aeration, and so on (Krull et al. 2013). It was discussed that properly controlled pellet size and inner structures allowed a reduced viscosity and facilitate oxygen mass transfer (Driouch et al. 2011). So far many related studies of morphological control focused on process optimization, and very little is known on the underlying molecular mechanisms and regulatory networks. Recently, studies using 95 morphology mutants of N. crassa revealed that improved cellulase production and protein secretion was achieved in pellet-forming mutants, but not in the mutants with long hyphae (Sun et al. 2014). Until now, no similar report on other filamentous fungi was found. It will be interesting to explore whether genetic engineering can be applied to finely modulate the morphology of filamentous fungi for efficient production of cellulosic enzymes.
6 Metabolic Engineering of Filamentous Fungi Using CRISPR-Cas9-Based Genome Editing System
The type II CRISPR (clustered regularly interspaced short palindromic repeats)-Cas9 (CRISPR-associated protein 9) system has recently been developed to enable rapid genome editing in various organisms (Doudna and Charpentier 2014), including various filamentous fungal species (Fuller et al. 2015; Liu et al. 2015; Matsu-Ura et al. 2015; Nødvig et al. 2015; Pohl et al. 2016; Liu et al. 2017).
The CRISPR-Cas9 system contains two components: the effector protein, which is the endonuclease Cas9, and a single chimeric guide RNA (sgRNA). In addition to Cas9, new effector protein Cpf1 was also developed and employed for genome editing (Zetsche et al. 2015). Although so far there is no report using Cpf1 in filamentous fungi, it is expected that novel effector proteins including Cpf1 will be utilized to improve efficiency of genome engineering of this important group of fungi. The sgRNA provides a 17–20 bp guide sequence that defines the target DNA, and the guide sequence was found adjacent a DNA motif (the PAM, protospacer adjacent motif) of three bases (NGG or TTN). The sgRNA binds to the effector protein and targets a specific locus in the recipient genome, where a double-strand break (DSB) will be introduced. The DSB can then be repaired by the host cell repair systems. In most cases, DSB is fixed by the error-prone non-homologous end-joining (NHEJ) mechanism (Fuller et al. 2015; Pohl et al. 2016). This can lead to random insertions or deletions within the target sequence. If a DNA share homology flanks closing to the DSB (a so-called donor DNA, dDNA) is available, homologous recombination (HR) will happen. And then the donor DNA can either replace or modify the target gene. Thus, the CRISPR systems can be used both for the deletion and insertion of genes (Pohl et al. 2016; Liu et al. 2017), resulting in marker-free gene disruption, deletion, or insertion (Doudna and Charpentier 2014).
The first example of genome editing in filamentous fungi was reported by Liu et al. (2015), which established the CRISPR-Cas9 system in T. reesei. Later on, such system was also successful in different filamentous fungal species, and Table 2.2 summarized related studies on cellulase-producing filamentous fungi, and the different genome editing methods were also briefly reviewed.
The efficient application of the CRISPR system requires the heterologous expression of the effector protein (Cas9 or Cpf1) fused to a nuclear localization signal (NLS) and simultaneous expression of the sgRNA (Schuster et al. 2016). For the expression of Cas9, two main strategies, in vitro and in vivo, have been established. In the case of in vitro, the Cas9-sgRNA ribonucleoprotein complex was formed in vitro to edit the target genes (Pohl et al. 2016). In the case of in vivo, the codon of Cas9 gene followed by a stronger NLS should be optimized for expression in filamentous fungi (Matsu-Ura et al. 2015; Fuller et al. 2015; Liu et al. 2015). In addition, different promoter systems also influence the expression of Cas9. When the strong constitutive promoters were used, the efficiency of genome editing should be improved; however, this way may lead to possible off-target effects. In order to realize the controllability of this system, some inducible promoters have been selected to inhibit Cas9 expression for minimal off-target effects (Liu et al. 2015; Pohl et al. 2016).
In addition to optimizing the expression of Cas9 gene, looking for optimal functional sgRNA modules was also very important (Schuster et al. 2016). The synthetic sgRNA needs to be transcribed using RNA polymerase III promoters (Nødvig et al. 2015). However, these promoters are poorly defined in filamentous fungi. For the expression of sgRNAs, two main strategies, in vitro and in vivo, have also been established. In some organisms, such as the T. reesei and A. niger, sgRNAs were generated in vitro and then co-transformed to the protoplasts together with a Cas9 gene expression cassette or the Cas9 protein (Zhang et al. 2016b). This strategy is suitable for almost all organisms. However, the stability of sgRNAs should be taken into consideration. It has been reported that RNA polymerase III promoters such as SNR52 and U6 and some tRNA promoters can be applied to transcribe sgRNAs in some of filamentous fungi, such as N. crassa and P. chrysogenum (Fuller et al. 2015; Matsu-Ura et al. 2015; Schuster et al. 2016). However, due to uncertainty of genome sequence and the complexity of genetic background, the endogenous RNA polymerase III promoters from filamentous fungi are difficult to be identified or not suitable for sgRNA transcription. Thus, the most common method to express sgRNAs in vivo is to use 5′-end hammerhead (HH) and 3′-end hepatitis delta virus (HDV) to flank the sgRNA (Nødvig et al. 2015), and then RNA polymerase II promoter can be used to express sgRNAs.
Compared with the traditional genetic engineering technology applied in filamentous fungi, CRISPR-Cas9 system has clear advantages. Firstly, it is simple. The same Cas9 can be used to target different genes, only sgRNA is different. Secondly, it is efficient. The CRISPR-Cas9 system not only can be used to rapidly manipulate single gene but also to modify multiple sites at the same time. Third, it is flexible. It enables marker-free engineering of the strain, therefore facilitating metabolic engineering of the strain by manipulating multiple genes. Fourth, it is not toxic. No difference of cell growth and sporulation was observed when cells were expressing Cas9 (Liu et al. 2017).
The disadvantage of the CRISPR-Cas9 system is its off-target effect. Therefore, precise genome editing will be developed, which can be achieved by high-fidelity CRISPR-Cas9 nucleases (Kleinstiver et al. 2016) and optimization of sgRNA design (Doench et al. 2016). Second, only deletion and replacement were achieved in filamentous fungi. Activation or repression of gene expression using this system as well as epigenome editing will also be pursued, which has been successful in mammalian system (Hilton et al. 2015; Konermann et al. 2015). It can be expected that the CRISPR-Cas9 system as well as similar genome editing method will be further improved in the near future in filamentous fungi, and functional genomic studies of filamentous fungi will be greatly promoted by these genome editing technologies, which will further enhance the efficiency of metabolic engineering manipulations.
7 Conclusion and Future Prospects
The economy of lignocellulosic biomass bioconversion is still hampered by high production cost of cellulase. In the past decades, significant progress has been made in understanding the induction and regulation of cellulase biosynthesis through functional genomic studies as well as multi-omics analysis. On the other hand, efficient genetic engineering methods have been developed to modify filamentous fungi, and these novel methods include marker-reuse techniques, promoter engineering, artificial transcription factor library, and genome editing using CRISPR-Cas9 systems. Development of systems biology and synthetic biology is providing new targets and concepts for construction of hyper producers for cellulase production.
Establishment of efficient genetic engineering platform of filamentous fungi is of great importance for development of hyper producing strains. Nowadays, most related reports only focused on single gene function; there is a lack of comprehensive metabolic engineering studies on filamentous fungi. It is expected that more progress of metabolic engineering of filamentous fungi will be made in the near future including but not being restricted in the below aspects:
-
1.
Functional genomic studies of filamentous fungi
It is vital to deepen the functional genomic studies of filamentous fungi, and various databases describing omics data, mutant phenotypes, function of transcription regulators, metabolites, and so on, should be developed for researchers to obtain detailed information for further functional genomic analysis. Metabolic engineering of filamentous fungi will rely on not only efficient genetic engineering and genome-editing methods, but also advanced techniques on analysis of gene expression and metabolites.
-
2.
Exploration of various genetic elements for metabolic engineering
These elements include promoters (both natural and synthetic ones), integration sites, selection markers, as well as transcription factors (both endogenous and artificial ones) for efficient metabolic engineering and synthetic biology design of filamentous fungi. The Cas9-based toolkit for different filamentous fungi will be developed, as has been achieved in the budding yeast S. cerevisiae (Reider et al. 2017).
-
3.
Development of genome editing techniques
The fast-growing knowledge on genome editing of mammalian cells will also lead to optimization of the genome editing techniques of filamentous fungi. So far most studies focused on metabolic engineering of filamentous fungi through transcriptional control of cellulase biosynthesis. Taking advantage of the CRISPR/dCas9 system, epigenetic regulation of cellulase production will also be manipulated to achieve precise control on chromatin dynamics.
The results obtained from the above mentioned studies will not only allow production of low-cost and efficient enzyme cocktails toward a better process economy, but also will lead to deeper understanding of the complex regulatory network of filamentous fungi, which is essential to further develop this important group of microorganisms as powerful microbial cell factories.
References
Ahuja M, Punekar NS (2008) Phosphinothricin resistance in Aspergillus niger and its utility as a selectable transformation marker. Fungal Genet Biol 45:1103–1110
Benocci T, Aguilar-Pontes MV, Zhou M et al (2017) Regulators of plant biomass degradation in ascomycetous fungi. Biotechnol Biofuels 10:152
Beri RK, Turner G (1987) Transformation of Penicillium chrysogenum using the Aspergillus nidulans amdS gene as a dominant selective marker. Curr Genet 11:639–641
Bischof RH, Ramoni J, Seiboth B (2016) Cellulases and beyond: the first 70 years of the enzyme producer Trichoderma reesei. Microb Cell Factories 15:106
Blazeck J, Garg R, Reed B et al (2012) Controlling promoter strength and regulation in Saccharomyces cerevisiae using synthetic hybrid promoters. Biotechnol Bioeng 109:2884–2895
Cao Y, Zheng F, Wang L et al (2017) Rce1, a novel transcriptional repressor, regulates cellulase gene expression by antagonizing the transactivator Xyr1 in Trichoderma reesei. Mol Microbiol 105:65–83
Castro LDS, Antoniêto ACC, Pedersoli WR et al (2014) Expression pattern of cellulolytic and xylanolytic genes regulated by transcriptional factors XYR1 and CRE1 are affected by carbon source in Trichoderma reesei. Gene Expr Patterns 14:88–95
Chakraborty BN (2015). Electroporation mediated DNA transformation of filamentous fungi. In: van den Berg MA, Maruthachalam K (eds) Genetic transformation systems in fungi, 1. Fungal biology. Springer, Cham pp 67–79
Chen L, Zou G, Wang J et al (2016) Characterization of the Ca2+-responsive signaling pathway in regulating the expression and secretion of cellulases in Trichoderma reesei Rut-C30. Mol Microbiol 100:560–575
Derntl C, Gudynaite-Savitch L, Calixte S et al (2013) Mutation of the xylanase regulator 1 causes a glucose blind hydrolase expressing phenotype in industrially used Trichoderma strains. Biotechnol Biofuels 6:62–72
Doench JG, Fusi N, Sullender M et al (2016) Optimized sgRNA design to maximize activity and minimize off-target of CRISPR-Cas9. Nat Biotechnol 34:184–191
Doudna J, Charpentier E (2014) Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346:1258096
Driouch H, Haensch R, Wucherpfennig T et al (2011) Improved enzyme production by bio-pellets of Aspergillus niger. Targeted morphology engineering using titanate microparticles. Biotechnol Bioeng 109:462–471
Fiedler MR, Gensheimer T, Kubisch C et al (2017) HisB as novel selection marker for gene targeting approaches in Aspergillus niger. BMC Microbiol 17:57
Forment JV, Ramón D, MacCabe AP (2006) Consecutive gene deletions in Aspergillus nidulans: application of the Cre/loxP system. Curr Genet 50(3):217–224
Frandsen RJN (2015) Agrobacterium tumefaciens-mediated transformation. In: van den Berg MA, Maruthachalam K (eds) Genetic transformation systems in fungi, vol 1. Springer, Cham, pp 143–152
Fuller KK, Chen S, Loros JJ et al (2015) Development of the CRISPR/Cas9 system for targeted gene disruption in Aspergillus fumigatus. Eukaryot Cell 14:1073–1080
Gao L, Gao F, Wang L et al (2012) N-glycoform diversity of cellobiohydrolase I from Penicillium decumbens and synergism of nonhydrolytic glycoform in cellulose degradation. J Biol Chem 287:15906–15915
Gruber F, Visser J, Kubicek CP et al (1990) The development of a heterologous transformation system for the cellulolytic fungus Trichoderma reesei based on a pyrG-negative mutant strain. Curr Genet 18:71–76
Gupta VK, Steindorff AS, de Paula RG et al (2016) The post-genomic era of Trichoderma reesei: what’s next? Trends Biotechnol 34:970–982
Hakkinen M, Arvas M, Oja M et al (2012) Re-annotation of the CAZy genes of Trichoderma reesei and transcription in the presence of lignocellulosic substrates. Microb Cell Factories 11:134
Hilton IB, D’Ippolito AM, Vockley CM et al (2015) Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat Biotechnol 33:510–517
Honda S, Selker EU (2009) Tools for fungal proteomics: multifunctional neurospora vectors for gene replacement, protein expression and protein purification. Genetics 182:11–23
Ivanova C, Ramoni J, Aouam T et al (2017) Genome sequencing and transcriptome analysis of Trichoderma reesei QM9978 strain reveals a distal chromosome translocation to be responsible for loss of vib1 expression and loss of cellulase induction. Biotechnol Biofuels 10:209
Jiang B, Zhang R, Feng D et al (2016) A Tet-on and Cre-loxP based genetic engineering system for convenient recycling of selection markers in Penicillium oxalicum. Front Microbiol 7:485
Kelly JM, Hynes MJ (1985) Transformation of Aspergillus niger by the amdS gene of Aspergillus nidulans. EMBO J 4:475–479
Kleinstiver BP, Pattanayak V, Prew MS et al (2016) High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature 529:490–495
Konermann S, Brigham MD, Trevino AE et al (2015) Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 517:583–588
Kopke K, Hoff B, Kuck U (2010) Application of the Saccharomyces cerevisiae FLP/FRT recombination system in filamentous fungi for marker recycling and construction of knockout strains devoid of heterologous genes. Appl Environ Microbiol 76:4664–4674
Krull R, Wucherpfennig T, Esfandabadi ME et al (2013) Characterization and control of fungal morphology for improved production performance in biotechnology. J Biotechnol 163:112–123
Kubicek CP, Mikus M, Schuster A et al (2009) Metabolic engineering strategies for the improvement of cellulase production by Hypocrea jecorina. Biotechnol Biofuels 2:19
Li C, Yang Z, Zhang RH et al (2013) Effect of pH on cellulase production and morphology of Trichoderma reesei and the application in cellulosic material hydrolysis. J Biotechnol 168:470–477
Li YH, Zhang XY, Xiong L et al (2017a) On-site cellulase production and efficient saccharification of corn Stover employing cbh2 overexpressing Trichoderma reesei with novel induction system. Bioresour Technol 238:643–649
Li Z, Liu G, Qu Y (2017b) Improvement of cellulolytic enzyme production and performance by rational designing expression regulatory network and enzyme system composition. Bioresour Technol S0960–8524(17):31024–31026
Lichius A, Seidl-Seiboth V, Seiboth B et al (2014) Nucleo-cytoplasmic shuttling dynamics of the transcriptional regulators XYR1 and CRE1 under conditions of cellulase and xylanase gene expression in Trichoderma reesei. Mol Microbiol 94:1162–1178
Liu G, Qin Y, Li Z et al (2013) Development of highly efficient, low-cost lignocellulolytic enzyme systems in the post-genomic era. Biotechnol Adv 31:962–975
Liu R, Chen L, Jiang Y et al (2015) Efficient genome editing in filamentous fungus Trichoderma reesei using the CRISPR/Cas9 system. Cell Discov 1:15007
Liu Q, Gao R, Li J et al (2017) Development of a genome-editing CRISPR/Cas9 system in thermophilic fungal Myceliophthora species and its application to hyper-cellulase production strain engineering. Biotechnol Biofuels 10(1):1
Ma L, Zhang J, Zou G et al (2011) Improvement of cellulase activity in Trichoderma reesei by heterologous expression of a beta-glucosidase gene from Penicillium decumbens. Enzym Microb Technol 49:366–371
Malmierca MG, Cardoza RE, Gutierrez S (2015) Trichoderma transformation methods. In: van den Berg MA, Maruthachalam K (eds) Genetics transformation systems in fungi, vol 1. Springer, Cham, pp 41–48
Matsu-Ura T, Baek M, Kwon J et al (2015) Efficient gene editing in Neurospora crassa with CRISPR technology. Fungal Biol Biotechnol 2:4
Mello-de-Sousa TM, Rassinger A, Pucher ME et al (2015) The impact of chromatic remodeling on cellulase expression in Trichoderma reesei. BMC Genomics 16:588
Nakari-Setala T, Paloheimo M, Kallio J et al (2009) Genetic modification of carbon catabolite repression in Trichoderma reesei for improved protein production. Appl Environ Microbiol 75:4853–4860
Nødvig CS, Nielsen JB, Kogle ME et al (2015) A CRISPR-Cas9 system for genetic engineering of filamentous fungi. PLoS One 10:e0133085
Noh W, Kim SW, Dong-Won B et al (2010) Genetic introduction of foreign genes to Pleurotus eryngii by restriction enzyme-mediated integration. J Microbiol 48:253–256
Novy V, Schmid M, Eibinger M et al (2016) The micromorphology of Trichoderma reesei analyzed in cultivations on lactose and solid lignocellulosic substrate, and its relationship with cellulase production. Biotechnol Biofuels 9:169
Peterson R, Nevalainen H (2012) Trichoderma reesei RUT-C30–thirty years of strain improvement. Microbiology 158:58–68
Pohl C, Kiel JA, Driessen AJ et al (2016) CRISPR/Cas9 based genome editing of Penicillium chrysogenum. ACS Synth Biol 5:754−764
Punt PJ, Oliver RP, Dingemanse MA et al (1987) Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene 56:117–124
Redden H, Alper HS (2015) The development and characterization of synthetic minimal yeast promoters. Nat Commun 6:7810
Reider Apel A, d’Espaux L, Wehrs M et al (2017) A Cas9-based toolkit to program gene expression in Saccharomyces cerevisiae. Nucleic Acids Res 45:496–508
Ruiz-Díez B (2002) Strategies for the transformation of filamentous fungi. J Appl Microbiol 92:189–195
Schmoll M, Tian C, Sun J et al (2012) Unravelling the molecular basis for light modulated cellulase gene expression-the role of photoreceptors in Neurospora crassa. BMC Genomics 13:127
Schuster M, Schweizer G, Reissmann S et al (2016) Genome editing in Ustilago maydis using the CRISPR–Cas system. Fungal Genet Biol 89:3–9
Seiboth B, Herold S, Kubicek CP (2012) Metabolic engineering of inducer formation for cellulase and hemicellulase gene expression in Trichoderma reesei. In: Wang XY, Chen J, Quinn P (eds) Reprogramming microbial metabolic pathways, Subcellular biotechnology, vol 64. Springer, Dordrecht, pp 367–390
Seidl V, Gamauf C, Druzhinina IS et al (2008) The Hypocrea jecorina (Trichoderma reesei) hypercellulolytic mutant RUT C30 lacks a 85 kb (29 gene-encoding) region of the wild-type genome. BMC Genomics 9:327
Steiger MG, Vitikainen M, Uskonen P et al (2011) Transformation system for Hypocrea jecorina (Trichoderma reesei) that favors homologous integration and employs reusable bidirectionally selection markers. Appl Environ Microbiol 77:114–121
Sternberg N, Hamilton D (1981) Bacteriophage P1 site-specific recombination: I recombination between loxP sites. J Mol Biol 150:467–486
Stricker AR, Grosstessner-Hain K, Wurleitner E et al (2006) Xyr1 (xylanase regulator 1) regulates both the hydrolytic enzyme system and D-xylose metabolism in Hypocrea jecorina. Eukaryot Cell 5:2128–2137
Stricker AR, Mach RL, De Graaff LH (2008) Regulation of transcription of cellulases-and hemicellulases-encoding genes in Aspergillus niger and Hypocrea jecorina (Trichoderma reesei). Appl Microbiol Biotechnol 78:211
Su X, Chu X, Dong Z (2009) Identification of elevated transcripts in a Trichoderma reesei strain expressing a chimeric transcription activator using suppression subtractive hybridization. World J Microbiol Biotechnol 25:1075–1084
Sun ZY, Lin LC, Wang M et al (2014) Improving cellulases production with Neurospora crassa by morphology mutants screening. Chin J Biotechnol 30:55–63
Unkles SE, Campbell EI, Carrez D et al (1989) Transformation of Aspergillus niger with the homologous nitrate reductase gene. Gene 78:157–166
Wang F, Liang Y, Wang M et al (2013a) Functional diversity of the p24γ homologue Erp reveals physiological differences between two filamentous fungi. Fungal Genet Biol 61:15–22
Wang S, Liu G, Wang J et al (2013b) Enhancing cellulase production in Trichoderma reesei RUT C30 through combined manipulation of activating and repressing genes. J Ind Microbiol Biotechnol 40:633–641
Wang S, Liu G, Yu J et al (2013c) RNA interference with carbon catabolite repression in Trichoderma koningii for enhancing cellulase production. Enzym Microb Technol 53:104–109
Wang W, Meng F, Liu P, Yang S et al (2014) Construction of a promoter collection for genes co-expression in filamentous fungus Trichoderma reesei J Ind Microbiol Biotechnol 41 (11):1709-1718
Xie GH, Wang XY, Ren LT (2010) China’s crop residues resources evaluation. Chin J Biotechnol 26:855–863
Xiong Y, Sun J, Glass NL (2014) VIB1, a link between glucose signaling and carbon catabolite repression, is essential for plant cell wall degradation by Neurospora crassa. PLoS Genet 10:e1004500
Yelton MM, Hamer JE, Timberlake WE (1984) Transformation of Aspergillus nidulans by using a trpC plasmid. Proc Natl Acad Sci USA 81:1470–1474
Yu L, Chao Y, Wensel P et al (2012) Hydrodynamic and kinetic study of cellulase production by Trichoderma reesei with pellet morphology. Biotechnol Bioeng 109:1755–1768
Zetsche B, Gootenberg JS, Abudayyeh OO et al (2015) Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 163:759–771
Zhang J, Zhong Y, Zhao X et al (2010) Development of the cellulolytic fungus Trichoderma reesei strain with enhanced β-glucosidase and filter paper activity using strong artificial cellobiohydrolase 1 promoter. Bioresour Technol 101:9815–9818
Zhang K, Pei Z, Wang D (2016a) Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: a review. Bioresour Technol 199:21–33
Zhang C, Meng X, Wei X et al (2016b) Highly efficient CRISPR mutagenesis by microhomology-mediated end joining in Aspergillus fumigatus. Fungal Genet Biol 86:47–57
Zhang F, Bai FW, Zhao XQ (2016c) Enhanced cellulase production from Trichoderma reesei Rut-C30 by engineering with an artificial zinc finger protein library. Biotechnol J 11:1282–1290
Zhang X, Li Y, Zhao X et al (2017) Constitutive cellulase production from glucose using the recombinant Trichoderma reesei strain overexpressing an artificial transcription activator. Bioresour Technol 223:317–322
Zhang F, Zhao XQ, Bai FW (2018) Improvement of cellulase production in Trichoderma reesei Rut-C30 by overexpression of a novel regulatory gene Trvib-1. Bioresour Technol 247:676–683
Zhao XQ, Xiong L, Zhang MM et al (2016) Towards efficient bioethanol production from agricultural and forestry residues: exploration of unique natural microorganisms in combination with advanced strain engineering. Bioresour Technol 215:84–91
Znameroski EA, Coradetti ST, Roche CM et al (2012) Induction of lignocellulose-degrading enzymes in Neurospora crassa by cellodextrins. Proc Natl Acad Sci USA 109:6012–6017
Zou G, Shi S, Jiang Y et al (2012) Construction of a cellulase hyper-expression system in Trichoderma reesei by promoter and enzyme engineering. Microb Cell Factories 11:21
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Zhao, XQ., Zhang, XY., Zhang, F., Zhang, R., Jiang, BJ., Bai, FW. (2018). Metabolic Engineering of Fungal Strains for Efficient Production of Cellulolytic Enzymes. In: Fang, X., Qu, Y. (eds) Fungal Cellulolytic Enzymes. Springer, Singapore. https://doi.org/10.1007/978-981-13-0749-2_2
Download citation
DOI: https://doi.org/10.1007/978-981-13-0749-2_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-0748-5
Online ISBN: 978-981-13-0749-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)