Abstract
Tooth number, shape, and position are consistent in mammals and are subject to strict genetic control. Multiple signaling pathways including Shh, Tgf, Bmp, Wnt, Fgf, Notch, and NF-kB are known to play critical roles in regulating tooth development. Recent studies show that these signaling pathways interact with each other through positive and negative feedback loops to regulate tooth number, shape, and spatial pattern. Teeth develop via a dynamic and complex reciprocal interaction between dental epithelium and cranial neural crest-derived mesenchyme. These interactions contain a series of inductive and permissive processes that lead to the determination, differentiation, and organization of odontogenic cells, which are controlled by these signaling pathways. It is believed that dozens of different molecules together form complex molecular networks that regulate tooth development. Studies of human congenital disease and transgenic mice suggest that disturbance of the molecular network results in abnormal tooth formation. Since molecular mechanisms involved in tooth development should be reproduced in tooth regeneration, knowledge of tooth development from both human and mouse studies is crucial for exploring tooth regenerative therapies. In this paper, we present an overview of the current literature covering the molecular mechanisms of tooth development, especially those regulating tooth number.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Tooth position, number, size, and shape are consistent in mammals and are determined genetically [6, 10, 13, 41, 80]. Teeth develop via sequential, reciprocal interactions between the oral epithelium and neural crest-derived mesenchyme. The first morphological sign of tooth development is an epithelial thickening (dental placode). The thickened tooth epithelium progressively takes the form of bud, cap, and bell configurations as differentiation and morphogenesis proceed. In addition to thickening of the epithelium, mesenchymal cells condense – a process that has been shown to be critical for organogenesis [44]. Subsequently, epithelial cells and mesenchymal cells (dental papilla) differentiate into enamel-producing ameloblasts and dentin-secreting odontoblasts, respectively. Since substantial research efforts over the last decade – using both human studies and transgenic mouse studies – have elucidated many aspects of the molecular mechanisms in tooth development, our understanding of the control of tooth shape diversity and location in the jaws has advanced considerably.
These research efforts have also elucidated that multiple signaling pathways including Shh, Tgf, Bmp, Wnt, Fgf, Notch, and NF-kB are known to play critical roles in regulating tooth development. The fine-tuning of these signaling pathways has been shown to be crucial in governing odontogenic precision. Recent studies show that crosstalk between these signaling pathways build complex molecular networks that regulate tooth development [6, 10, 13, 26, 28, 41, 80].
Regenerative medicine is one of the revolutionary future therapies in dentistry. Since regeneration of organs starts from initiation, knowledge of the molecular mechanisms involved at this stage is crucial for developing tooth regenerative therapies. Missing and extra teeth have been shown to be caused by disturbance of the developmental mechanisms during the initiation stage. Studying these numerical anomalies in both humans and mice therefore provides invaluable information to understanding the molecular mechanisms of tooth initiation and therefore tooth regeneration. In this paper, we present an overview covering the molecular mechanisms of tooth development, especially those determining tooth number.
2 Skin Appendage
The skin serves several functions, including thermoregulation, protection from the external environment, maintaining internal tissue fluid from evaporation, sensation, defense against infection, and supporting scaffold for hair. To fulfill these multiple functions, skin develops many structures as epidermal appendages such as nails, sweat glands, hair, sebaceous glands, tooth, and mammary glands. Although diverse in their structural phenotypes, these organs including teeth and hair share common morphological features in the early stages of development – i.e., epithelial components originate as thickenings that subsequently form buds around which the underlying mesenchymal cells condense. Interactions between the epithelial and mesenchymal tissues play central roles in regulating the morphogenesis of the skin appendages. When cultured alone, neither the epithelial nor mesenchymal components of these structures can differentiate into specific cells. It is also known that epithelial-mesenchymal interactions are sequential and reciprocal occurring in both directions between the epithelial and mesenchymal tissues.
Mice are the most highly studied mammals for investigating the mechanisms of tooth development. Initiation begins before the organ anlagen are morphologically visible. The first odontogenic signals derive from the tooth epithelium. Bmps-, Fgfs-, Wnts-, NF-kB-, and Shh-related genes are expressed in the presumptive tooth epithelium before the thickening process. Unlike humans, rodent incisors grow continuously throughout life and the stem cell niche is known to be located at the apical end of the incisor tooth. In the laboratory, hair can be initiated from murine dental stem cells, suggesting that tooth epithelium also retains the ability to form hair [84].
3 Missing Teeth
3.1 Missing Teeth in Humans
Congenital dental anomalies can occur either as non-syndromic familial cases or as part of a syndrome. Missing teeth are one of the most common human developmental anomalies. It has been shown that more than 20 % of humans lose at least one of the third molar teeth [36, 45, 53]. 1.6–9.6 % of the population suffers from hypodontia, with loss of one or two teeth (except the third molar). Oligodontia, with loss of more than six teeth (except the third molar), ranges from 0.0 % to 1.1 %, depending on the population studied. Loss of all teeth is referred to as anodontia. Missing teeth are more common in the permanent dentition than those in the primary dentition. However, it is believed that hypodontia in the primary dentition is correlated to hypodontia in the permanent dentition [36, 45]. Additionally, a higher prevalence ratio of hypodontia has been identified in women, with a 3:2 ratio [9].
3.1.1 Non-syndromic (Isolated) Familial Missing Teeth
Non-syndromic familial hypodontia has been reported to probably be inherited either as an autosomal dominant, autosomal recessive, or X-linked trait [1, 2, 15, 22, 65, 79].
It has been shown that mutation in MSX1 is associated with hypodontia that predominantly affects second premolars and third molars which all have normal primary dentition [31, 45]. Mutation in PAX9 results in hypodontia in the form of lost molars. Maxillary and/or mandibular second molars and central incisor are often affected in some individuals [37, 46]. The frequency of tooth loss is found to be higher for second premolars and maxillary first premolars in association with MSX1 mutation compared to PAX9 mutation [53]. Each tooth type thus shows their different response to these gene mutations. Defects identified in MSX1 and PAX9 include gene deletion, as well as nonsense, frameshift, and missense mutation. In addition, it has been indicated that a nonsense mutation in AXIN2 (a molecule essential for canonical Wnt signaling) leads to oligodontia [40]. Here, patients display an absence of at least eight permanent teeth, while primary dentition remains intact.
Mutation in EDA has been shown to be linked to non-syndromic hypodontia, although mutation in EDA can also lead to syndromic dental anomalies [83]. Mutation of WNT10A is found in patients with isolated hypodontia, even though mutation of the gene is also linked to odonto-onycho-dermal dysplasia [77]. Additionally, GREM2 mutations have been found to exist within families with absent teeth [30].
3.1.2 Syndromic Missing Teeth
There are about eighty syndromes showing hypodontia (Table 13.1; [36]).
Ectodermal dysplasia consists of variable defects in the morphogenesis of ectodermal derivatives such as teeth, skin, hair, sweat glands, and nails, although more than 150 clinically distinct ectodermal dysplasias have been identified. Ectodermal dysplasia syndromes can be inherited in an autosomal dominant, autosomal recessive, or X-linked form. Disruption of the EDA, EDAR, and EDARADD loci has been causally identified for the onset of hypohidrotic/anhidrotic ectodermal dysplasia [56]. Odonto-onycho-dermal dysplasia is an autosomal recessive ectodermal syndrome, which is caused by mutation in WNT10A. It is characterized by hyperkeratosis, smooth tongue, dry hair, nail dysplasia, and hyperhidrosis of palms and soles. The dysplasia also presents with severe hypodontia [36]. Ectrodactyly ectodermal dysplasia cleft lip/palate syndrome is an autosomal dominant disorder characterized by ectrodactyly, ectodermal dysplasia, and cleft lip/palate. Patients with this syndrome also exhibit oligodontia/anodontia. Heterozygous mutation in P63 has additionally been shown to be responsible for ectrodactyly ectodermal dysplasia cleft lip/palate syndrome [73]. Incontinentia pigmenti is an X-linked dominant disorder, which is characterized by abnormal skin pigmentation and hair loss. It is caused by mutation of IKKγ (NEMO) [56]. Incontinentia pigmenti is recognized as a form of ectodermal dysplasia by many societies, since ectodermal structures (hair, skin, nails, and teeth) as well as the eyes and nervous system are affected. Hypodontia is a common feature of the disorder [73].
Ellis-van Creveld syndrome is characterized by polydactyly, chondrodysplasia, nail dysplasia, and cardiac defects and is caused by mutation of EVC. Missing teeth are frequently observed in Ellis-van Creveld syndrome patients [5]. Van der Woude syndrome is characterized by cleft lip and/or cleft palate, paramedian lip pits and sinuses, and conical elevations of the lower lip. It is caused by mutation in the IRF6 gene. Hypodontia is a common feature of the syndrome [55, 72]. Rieger syndrome is an autosomal dominant disorder, which consists of malformations in the anterior chamber of the eye and umbilical anomalies. Rieger syndrome patients also show hypodontia or anodontia. Mutations in PITX2 and FOXC1 have additionally been shown to be responsible for Rieger syndrome. Oral facial digital syndrome type I is an X-linked disorder which is caused by mutation of OFD1. The syndrome is characterized by malformation of the face, oral cavity, digits, central nervous system, and kidneys. Missing teeth are often observed in oral facial digital syndrome type I patients [23]. Enamel renal gingival syndrome is caused by the mutation of FAM20, and patients display impaired calcium metabolism and hypodontia [29].
It is generally accepted that hypodontia is often accompanied by clefts of the lip and palate. Hypodontia has been shown to be observed in 80 % of patients with clefts [36, 74]. The prevalence ratio of hypodontia is also correlated to the severity of the cleft [69].
3.1.3 Sporadic Missing Teeth
It is believed that sporadic hypodontia is caused by environmental factors such as trauma to the jaws, surgical procedures on the jaws, traumatic extraction of the primary teeth, chemotherapy, and radiation therapy [52, 71]. In common with environmental factors, mutation of PAX9 has been shown to be associated with sporadic hypoplasia [62].
3.2 Missing Teeth in Mice
Mice are the most highly studied mammals for investigating the molecular mechanisms of tooth development, since the most common application of gene targeting is to produce knockout mice. Mice however only have incisor and molar teeth and as such their use is limited as a model for human tooth development.
The first odontogenic signal is derived from the epithelium. Prior to this, there is no prespecification of the cells into different populations within the mandibular arch. All ectomesenchyme cells in the arch are therefore equally responsive toward any signals, including those which instigate hair and limb development. After receiving appropriate signaling, the underlying mesenchyme becomes independent of the epithelial cues. From the bud stage, the direction of cellular communication is reversed and signals pass from the condensing mesenchyme to the epithelium.
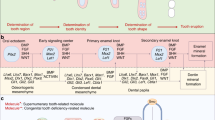
Many genes have been shown to play a critical role in regulating tooth number (Table 13.2). In common with humans, mice with mutations in Msx1 and Pax9 mutation exhibit missing teeth, driven by an arrest of tooth development at the bud stage [64, 70]. Mutation of other molecules such as Fgfr2, Lef1, and Runx2 also shows an arrest of tooth development at the bud stage [16, 18, 78]. Transition to cap stage from bud stage is thus a critical point in tooth development. In common with humans, p63 and Pitx2 mutant mice show missing teeth, but this is caused by an arrest of tooth development at stages preceding the bud stage [39, 42, 43]. Together, these data suggest that p63 and Pitx2 are essential for developmental signaling before the requirement of Msx1, Pax9, Fgfr2, Lef1, and Runx2. However Msx1/2 mutants also show an arrest of tooth development at stages preceding the bud stage, suggesting that Msx1 has a role in tooth initiation for which Msx2 can compensate for gene loss in the mutant mouse [7].
It is believed that Shh, Tgf, Bmp, Wnt, and Fgf signaling are known to play critical roles in tooth initiation. Gli2/3 (transcription factors mediated by Shh) mutants show an arrest of tooth development at stages prior to bud formation [24]. Mice with epithelial conditional deletion of Bmpr1a lead to an arrest of tooth development at the bud stage [4]. Additionally, Fgf3/10 mutants show an arrest of tooth development preceding the bud stage, whereas mutation of Fgfr2 results in an arrest of tooth development at the bud stage [16, 82]. Downregulation of Wnt signaling by overexpression of Dkk1 (an antagonist of Wnt signaling) shows an arrest of tooth development at stages prior to bud formation, whereas Lef1 (a Wnt-related molecule) mutation results in an arrest of tooth development at the bud stage [3, 78]. In common with Msx1 and Msx2, it is likely that there is redundancy between molecules within the same signaling pathway during tooth initiation events.
The term “epigenetics” refers to the covalent modification of DNA, protein, or RNA, resulting in changes to the function and/or regulation of these molecules without altering the genomic sequence. MicroRNAs (miRNAs) represent one of these epigenetic factors. These noncoding small single-stranded RNAs are 19–25 nucleotides in length and negatively regulate gene expression by binding target sequences in mRNA molecules. Absence of miRNAs in neural crest-derived cells, driven by deletion of Dicer (an essential molecule for microRNA processing), have been shown to lead to an absence of tooth development – although exact phenotypes vary between animals from lack of tooth development to almost normal tooth development [61]. This suggests that epigenetic factors also play a critical role in the regulation of tooth formation, but the exact mechanism is as yet unclear.
4 Supernumerary Teeth
Teeth are found in most vertebrates and have played a central role in their evolution. Change in tooth number is a significant evolutionary adaptation to accommodate novel feeding strategies. Reduction in tooth number is a well-known evolutionary trend of the dentition within eutherians. The total number of teeth per dentition has generally decreased, whereas tooth morphological complexity has increased during tooth evolution. Findings in tooth evolution are therefore a key feature to understanding the molecular mechanisms in regulating tooth number.
4.1 Supernumerary Teeth in Humans
The prevalence ratio of supernumerary teeth ranges from 0.2 % to 0.8 % in the deciduous dentition and from 0.3 % to 5.3 % in the permanent dentition [80]. The incidence of supernumerary teeth in men is higher than those in women (1.8:1–4.5:1, female/male) [53, 80].
4.1.1 Syndromic Extra Teeth in Humans
Multiple supernumerary teeth are usually a syndromic symptom, and the prevalence for non-syndromic multiple supernumerary teeth have been reported to be less than 1 % (Table 13.1; [68]).
Cleidocranial dysplasia (dysostosis) is characterized by general bone dysplasia, short stature, delayed closure of the cranial sutures, and hypoplastic or aplastic clavicles. Cleidocranial dysplasia is known to be associated with supernumerary dentition. Mutations in RUNX2 are responsible for the dysplasia [36, 80]. SOX2 anophthalmia syndrome is caused by mutations in the SOX2 gene and is characterized by anophthalmia or microphthalmia, with various extraocular symptoms such as hypogonadotropic hypogonadism, brain anomaly, and esophageal abnormalities. Again, patients show supernumerary tooth formation [54]. Gardner syndrome is a rare autosomal dominant disorder which is caused by mutations in APC. The syndrome consists of gastrointestinal polyps, multiple osteomas, and skin and soft tissue tumors including a characteristic retinal lesion. Variant tooth anomalies including supernumerary teeth are observed in these patients. In addition, Opitz G/BBB syndrome, tricho-rhino phalangic syndrome, Ehlers-Danlos syndrome type III, Robinow syndrome, Nance-Horan syndrome, Fabry syndrome, Rothmund-Thomson syndrome, and Hallermann-Streiff syndrome are all known to be syndromes where supernumerary teeth occur [13, 73, 80].
As with hypodontia, syndromic extra tooth formation is often accompanied by cleft lip/palate. In this instance, splitting of the tooth germs due to cleft lip and/or palate is the cause of the formation of supernumerary teeth [38, 80].
4.1.2 Missing and Extra Teeth in Humans
The concomitant occurrence of hypodontia and supernumerary teeth is observed in Down syndrome, oral facial digital syndrome type I, Ellis-van Creveld syndrome, and Ehlers-Danlos syndrome [76]. These suggest that single gene mutation can lead to these opposite tooth phenotypes in same patients.
4.2 Supernumerary Teeth in Mice
It is widely accepted that modern eutherian mammals evolved from a common ancestor that had three incisors, one canine, four premolars, and three molars. Mice possess only one incisor and three molars in each jaw quadrant, separated by a toothless region called the “diastema.” It is believed that mice lost the remaining teeth during evolution. Genetically modified mice therefore provide some limited information on the determinant of non-murine tooth types. However, it has been shown that mice have retained the genetic potential for the development of teeth lost during evolution, such as the premolars.
In wild-type mice, tooth germ-like structures are observed in the diastema at early stages of development, which disappear during later stages [63]. Both mutation and overexpression of Eda are known to lead to extra tooth formation in the diastema, suggesting that the precise signaling regulated by Eda is essential for controlling odontogenesis in the diastema [48, 63]. Extra tooth formation in the diastema is also observed in mice with mutation of Gas1 (an inhibitor of Shh), R-spondin2 (activators of Wnt signaling), Ift88 (molecules present in the primary cilia where Shh signaling is activated), Wise (a secreted BMP antagonist and Wnt modulator), Lrp4 (a negative Wnt co-receptor), Sprouty2 (a negative feedback regulator of Fgf), and Sprouty4 (a negative feedback regulator of Fgf) [32, 33, 35, 59, 60]. Evolutionary tooth loss in the diastema is likely to be associated with changes in these signaling pathways. These also suggest that odontogenic activity in the diastema is mainly controlled by inhibitors of signaling pathways.
Mammals have single-rowed dentitions, whereas many other vertebrates have dentition which consists of multiple rows. It is believed that mammals lost this additional dentition during evolution. Osr2 is expressed in the molar tooth germ area with a lingual-to-buccal gradient and restricts expression of Bmp4 – which is also mediated by Msx1. Mice with mutations in Osr2 develop supernumerary teeth lingual to the endogenous molars due to expansion of Bmp4 expression [85]. The interaction between Osr2, Bmp4, and Msx1 thus plays a critical role in regulating molar tooth initiation in the buccolingual axis [41].
In common with the diastema and molar region, extra incisor tooth formation is also observed in mice with mutations in Wise, Lrp4, Lhx6/7 and Sprouty2/4, and overexpressing Ikkβ [8, 11, 17, 32, 59]. Single extra incisor in a jaw quadrant is thus formed by changes in the Wnt, Bmp, Fgf, and NF-kB pathways.
Constitutive stabilization of β-catenin in the epithelium results in numerous supernumerary tooth formation in the mouse [25]. Apc is known to play a critical role in regulating the Wnt signaling pathway and conditional deletion of Apc in the epithelium also results in multiple supernumerary tooth formation [81]. Mice with overexpression of Lef1 (a Wnt-related molecule) have been associated with ectopic tooth formation [86]. Numerous extra teeth are also observed in Epiprophin (Sp6)-deficient mice, which show upregulation of Wnt signaling [49]. Taken together, numerous extra tooth formation is thus directly related to over-activation of Wnt signaling. Wnt inhibitors, Dkks, are known to be expressed in wild-type developing tooth germs, suggesting that Wnt signaling activity is regulated by the balance between ligands and inhibitors [21].
5 Odontogenic Activity Between Tooth Germs
Each tooth type is known to show different developmental timing in humans. In mice, the second and third molars start to develop after the first molar reach the bell stage. It has been shown that the first molar tooth germs inhibit development of the second molar at an early stage [34]. The development of the second molar tooth initiates only when inhibitory factors from the first molar are sufficiently reduced. Odontogenic activity is thus regulated by the interaction between tooth germs. Bmp signaling is likely to be involved in this interaction. Indeed, mice with mesenchymal conditional deletion of Bmp4 and mice overexpressing Noggin (Bmp antagonist) present with an absence of second and/or third molars [27, 67]. Mice with mutation of Eda and with reduction of NF-kB also exhibit loss of the second and/or third molars, suggesting that Eda-NF-kB cascade is also involved in the interaction between tooth germs [57, 66].
6 Tooth Initiation and Tooth Type
It has been shown that more than 20 % of humans lose at least one of the third molars [36, 45, 53]. Apart from the third molars, lower second premolars and/or upper lateral incisors are most commonly affected, followed by the upper second premolars [53]. Most of the common supernumerary teeth (46.9–92.8 % of supernumerary teeth) are observed between the upper central incisors – the so-called mesiodens [20, 68, 87]. Supernumerary teeth are also observed in the premolar region (10 % of the total supernumerary cases) and almost 75 % of those are in the mandible [80]. Supernumerary teeth are also found in the molar region as a distomolar (fourth molar). Furthermore, mice with targeted null mutations of both Dlx1 and Dlx2 homeobox genes have a tooth patterning phenotype where development of maxillary molar teeth is inhibited but development of all other teeth is normal. ActivinβA mutants show the opposite tooth phenotype, where maxillary molar teeth are present and other teeth are absent [19]. Odontogenic activities are thus differently regulated between regions of the maxillary and mandibular jaw.
It has been established that instructive signals are involved in the determination of tooth type. Homeobox genes have been found to regulate patterning in the development of many tissues including the maxillae and mandibles. In the developing jaws, several homeobox genes show highly restricted expression patterns in the ectomesenchyme along the proximodistal axis. For example, Barx1 are expressed in mesenchymal cells of the presumptive molar region, whereas Msx1 and Msx2 are expressed in mesenchymal cells where the incisors develop. Spatially restricted gene expression is also observed in the epithelium. Bmp4 is expressed in the presumptive incisor region, while Fgf8 expression is restricted to the presumptive molar region. In the epithelium, these molecules have been shown to be responsible for regulating mesenchymal homeobox gene expression. Mis-expression of Barx1 in the murine presumptive incisor mesenchyme results in a transformation of tooth shape, with molars developing from incisor primordia. Transposition of teeth (i.e., adjacent teeth switching positions) is an extremely rare dental anomalies in humans [12, 75]. However, examples of molar-like teeth in the maxillary central incisor region and premolar-like teeth in the maxillary lateral incisor region have previously been reported [28]. It is believed that the maxillary canine is most frequently involved in the transposition event [51]. Tooth identity is believed to be determined by complicated mechanisms, such as spatially restricted homeobox gene expression, and the gradient and overlap of signaling molecules [13, 14, 47]. Although the etiology of transposed teeth remains unclear, it is possible that changes in the balance of the determinant molecules results in the switching of tooth types.
7 The Midline and Tooth Development
Holoprosencephaly is a relatively common defect of the forebrain and midface in humans and is caused by impaired midline cleavage of the embryonic forebrain. It is believed that the holoprosencephaly spectrum is associated with the appearance of a solitary median maxillary central incisor. Single maxillary central incisors are also a feature of single median maxillary central incisor syndrome, which is caused by a failure in growth at the midline [10, 50]. Craniofacial development is thus linked to tooth development.
8 Conclusion
Tooth number, shape, and position are consistent in mammals and are subject to strict genetic control. Here, we highlight an overview covering the molecular mechanisms of tooth development, especially those regulating tooth number. Dozens of different molecules together form complex molecular networks creating positive and negative feedback loops, and a series of inductive and permissive processes that regulate tooth number. Multiple signaling pathways such as Shh, Tgf, Bmp, Wnt, Fgf, Notch, and NF-kB – and crosstalk between them – are known to play critical roles in regulating tooth development. Activity of signaling pathways is also regulated by the balance of ligands, activators, inhibitors, and receptors. Teeth develop via a dynamic and complex reciprocal interaction between dental epithelium and cranial neural crest-derived mesenchyme. It has been shown that transcription factors are involved in epithelial-mesenchymal interactions through the signaling loops between tissue layers by responding to inductive signals and regulating the expression of other signaling molecules. It has been shown that all these factors function as distinct role between tooth type, timing, location, and gender in mammals.
Studies of human congenital disease and transgenic mice suggest that disturbance of the molecular network results in abnormal tooth formation. Since molecular mechanisms involved in tooth development should be reproduced in tooth regeneration, knowledge of tooth development from both human and mouse studies provides crucial information for the advancement of tooth regenerative therapy. Among the molecular mechanisms involved in tooth development, those regulating tooth number are the most critical for tooth regeneration, as replacement dentition should start from tooth initiation. Rodent incisors grow continuously throughout life by utilizing a stem cell niche located at the apical end of the incisor tooth. As such, this structure is also able to provide crucial information pertinent to the study of tooth regeneration.
References
Ahmad W, Brancolini V, UL Faiyaz MF, Lam H, UL Haque S, Haider M, Maimon A, Aita VM, Owen J, Brown D, Zegarelli DJ, Ahmad M, Ott J, Christiano AM. A locus for autosomal recessive hypodontia with associated dental anomalies maps to chromosome 16q12.1. Am J Hum Genet. 1998;62:987–91.
Alvesalo L, Portin P. The inheritance pattern of missing, peg-shaped, and strongly mesio-distally reduced upper lateral incisors. Acta Odontol Scand. 1969;27:563–75.
Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–53.
Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, Croft NJ, Cebra-Thomas JA, Metzger D, Chambon P, Lyons KM, Mishina Y, Seykora JT, Crenshaw 3rd EB, Millar SE. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–68.
Baujat G, Le Merrer M. Ellis-van Creveld syndrome. Orphanet J Rare Dis. 2007;2:27.
Bei M. Molecular genetics of ameloblast cell lineage. J Exp Zool B Mol Dev Evol. 2009;312B(5):437–44.
Bei M, Maas R. FGFs and BMP4 induce both Msx1-independent and Msx1-dependent signaling pathways in early tooth development. Development. 1998;125:4325–33.
Blackburn J, Kawasaki K, Porntaveetus T, Kawasaki M, Otsuka-Tanaka Y, Miake Y, Ota MS, Watanabe M, Hishinuma M, Nomoto T, Oommen S, Ghafoor S, Harada F, Nozawa-Inoue K, Maeda T, Peterková R, Lesot H, Inoue J, Akiyama T, Schmidt-Ullrich R, Liu B, Hu Y, Page A, Ramírez A, Sharpe PT, Ohazama A*. Excess NF-kB induces ectopic odontogenesis in embryonic incisor epithelium. J Dent Res. 2015;94:121–8.
Brook AH. Dental anomalies of number, form and size: their prevalence in British schoolchildren. J Int Assoc Dent Child. 1974;5:37–53.
Catón J, Tucker AS. Current knowledge of tooth development: patterning and mineralization of the murine dentition. J Anat. 2009;214:502–15.
Charles C, Hovorakova M, Ahn Y, Lyons DB, Marangoni P, Churava S, Biehs B, Jheon A, Lesot H, Balooch G, Krumlauf R, Viriot L, Peterkova R, Klein OD. Regulation of tooth number by fine-tuning levels of receptor-tyrosine kinase signaling. Development. 2011;138:4063–73.
Chattopadhyay A, Srinivas K. Transposition of teeth and genetic etiology. Angle Orthod. 1996;66:147–52.
Cobourne MT, Sharpe PT. Making up the numbers: the molecular control of mammalian dental formula. Semin Cell Dev Biol. 2010;21:314–24.
Cobourne MT, Mitsiadis T. Neural crest cells and patterning of the mammalian dentition. J Exp Zool B Mol Dev Evol. 2006;306:251–60.
De Coster PJ, Marks LA, Martens LC, Huysseune A. Dental agenesis: genetic and clinical perspectives. J Oral Pathol Med. 2009;38:1–17.
De Moerlooze L, Spencer-Dene B, Revest JM, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127:483–92.
Denaxa M, Sharpe PT, Pachnis V. The LIM homeodomain transcription factors Lhx6 and Lhx7 are key regulators of mammalian dentition. Dev Biol. 2009;333:324–36.
D’Souza RN, Aberg T, Gaikwad J, Cavender A, Owen M, Karsenty G, Thesleff I. Cbfa1 is required for epithelial–mesenchymal interactions regulating tooth development in mice. Development. 1999;126:2911–20.
Ferguson CA, Tucker AS, Christensen L, Lau AL, Matzuk MM, Sharpe PT. Activin is an essential early mesenchymal signal in tooth development that is required for patterning of the murine dentition. Genes Dev. 1998;12:2636–49.
Fernández Montenegro P, Valmaseda Castellón E, Berini Aytés L, Gay Escoda C. Retrospective study of 145 supernumerary teeth. Med Oral Patol Oral Cir Bucal. 2006;11:E339–44.
Fjeld K, Kettunen P, Furmanek T, Kvinnsland IH, Luukko K. Dynamic expression of Wnt signaling-related Dickkopf1, -2, and -3 mRNAs in the developing mouse tooth. Dev Dyn. 2005;233(1):161–6.
Goldenberg M, Das P, Messersmith M, Stockton DW, Patel PI, D’Souza RN. Clinical, radiographic, and genetic evaluation of a novel form of autosomal-dominant oligodontia. J Dent Res. 2000;79:1469–75.
Gurrieri F, Franco B, Toriello H, Neri G. Oral-facial-digital syndromes: review and diagnostic guidelines. Am J Med Genet A. 2007;143A:3314–23.
Hardcastle Z, Mo R, Hui CC, Sharpe PT. The Shh signalling pathway in tooth development: defects in Gli2 and Gli3 mutants. Development. 1998;125:2803–11.
Järvinen E, Salazar-Ciudad I, Birchmeier W, Taketo MM, Jernvall J, Thesleff I. Continuous tooth generation in mouse is induced by activated epithelial Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A. 2006;103:18627–32.
Jheon AH, Seidel K, Biehs B, Klein OD. From molecules to mastication: the development and evolution of teeth. Wiley Interdisc Rev Dev Biol. 2013;2:165–82.
Jia S, Zhou J, Gao Y, Baek JA, Martin JF, Lan Y, Jiang R. Roles of Bmp4 during tooth morphogenesis and sequential tooth formation. Development. 2013;140:423–32.
Kantaputra PN, Gorlin RJ. Double dens invaginatus of molarized maxillary central incisors, premolarization of maxillary lateral incisors, multituberculism of the mandibular incisors, canines and first premolar, and sensorineural hearing loss. Clin Dysmorphol. 1992;1:128–36.
Kantaputra PN, Bongkochwilawan C, Kaewgahya M, Ohazama A, Kayserili H, Erdem AP, Aktoren O, Guven Y. Enamel-renal-gingival syndrome, hypodontia, and a novel FAM20A mutation. Am J Med Genet A. 2014;164A:2124–8.
Kantaputra PN, Kaewgahya M, Hatsadaloi A, Vogel P, Kawasaki K, Ohazama A. Ketudat Cairns JR6. GREMLIN 2 mutations and dental anomalies. J Dent Res. 2015;94:1646–52.
Kapadia H, Mues G, D’Souza R. Genes affecting tooth morphogenesis. Orthod Craniofac Res. 2007;10:237–44.
Kassai Y, Munne P, Hotta Y, Penttila E, Kavanagh K, Ohbayashi N, Takada S, Thesleff I, Jernvall J, Itoh N. Regulation of mammalian tooth cusp patterning by ectodin. Science. 2005;309:2067–70.
Kawasaki M, Porntaveetus T, Kawasaki K, Oommen S, Otsuka-Tanaka Y, Hishinuma M, Nomoto T, Maeda T, Takubo K, Suda T, Sharpe PT, Ohazama A. R-spondins/Lgrs expression in tooth development. Dev Dyn. 2014;243:844–51.
Kavanagh KD, Evans AR, Jernvall J. Predicting evolutionary patterns of mammalian teeth from development. Nature. 2007;449:427–32.
Klein OD, Minowada G, Peterkova R, Kangas A, Yu BD, Lesot H, Peterka M, Jernvall J, Martin GR. Sprouty genes control diastema tooth development via bidirectional antagonism of epithelial-mesenchymal FGF signaling. Dev Cell. 2006;11:181–90.
Klein OD, Oberoi S, Huysseune A, Hovorakova M, Peterka M, Peterkova R. Developmental disorders of the dentition: an update. Am J Med Genet C Semin Med Genet. 2013;163C:318–32.
Kobielak A, Kobielak K, Wiśniewski AS, Mostowska A, Biedziak B, Trzeciak WH. The novel polymorphic variants within the paired box of the PAX9 gene are associated with selective tooth agenesis. Folia Histochem Cytobiol. 2001;39:111–2.
Kriangkrai R, Chareonvit S, Yahagi K, Fujiwara M, Eto K, Iseki S. Study of Pax6 mutant rat revealed the association between upper incisor formation and midface formation. Dev Dyn. 2006;235:2134–43.
Laurikkala J, Mikkola ML, James M, Tummers M, Mills AA, Thesleff I. p63 regulates multiple signalling pathways required for ectodermal organogenesis and differentiation. Development. 2006;133:1553–63.
Lammi L, Arte S, Somer M, Jarvinen H, Lahermo P, Thesleff I, Pirinen S, Nieminen P. Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. Am J Hum Genet. 2004;74:1043–50.
Lan Y, Jia S, Jiang R. Molecular patterning of the mammalian dentition. Semin Cell Dev Biol. 2014;25–26:61–70.
Lin CR, Kioussi C, O’Connell S, Briata P, Szeto D, Liu F, Izpisua-Belmonte JC, Rosenfeld MG. Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature. 1999;401:279–82.
Lu MF, Pressman C, Dyer R, Johnson RL, Martin JF. Function of Rieger syndrome gene in left-right asymmetry and craniofacial development. Nature. 1999;401:276–8.
Mammoto T, Mammoto A, Torisawa YS, Tat T, Gibbs A, Derda R, Mannix R, de Bruijn M, Yung CW, Huh D, Ingber DE. Mechanochemical control of mesenchymal condensation and embryonic tooth organ formation. Dev Cell. 2011;21:758–69.
Matalova E, Fleischmannova J, Sharpe PT, Tucker AS. Tooth agenesis: from molecular genetics to molecular dentistry. J Dent Res. 2008;87:617–23.
Mensah JK, Ogawa T, Kapadia H, Cavender AC, D’Souza RN. Functional analysis of a mutation in PAX9 associated with familial tooth agenesis in humans. J Biol Chem. 2004;279:5924–33.
Mitsiadis TA, Smith MM. How do genes make teeth to order through development? J Exp Zool B Mol Dev Evol. 2006;306:177–82.
Mustonen T, Pispa J, Mikkola ML, Pummila M, Kangas AT, Pakkasjärvi L, Jaatinen R, Thesleff I. Stimulation of ectodermal organ development by Ectodysplasin-A1. Dev Biol. 2003;259:123–36.
Nakamura T, de Vega S, Fukumoto S, Jimenez L, Unda F, Yamada Y. Transcription factor epiprofin is essential for tooth morphogenesis by regulating epithelial cell fate and tooth number. J Biol Chem. 2008;283:4825–33.
Nanni L, Ming JE, Du Y, Hall RK, Aldred M, Bankier A, Muenke M. SHH mutation is associated with solitary median maxillary central incisor: a study of 13 patients and review of the literature. Am J Med Genet. 2001;102:1–10.
Nambiar S, Mogra S, Shetty S. Transposition of teeth: a forensic perspective. J Forensic Dent Sci. 2014;6:151–3.
Näsman M, Forsberg CM, Dahllöf G. Long-term dental development in children after treatment for malignant disease. Eur J Orthod. 1997;19:151–9.
Nieminen P. Genetic basis of tooth agenesis. J Exp Zool B Mol Dev Evol. 2009;312B:320–42.
Numakura C, Kitanaka S, Kato M, Ishikawa S, Hamamoto Y, Katsushima Y, Kimura T, Hayasaka K. Supernumerary impacted teeth in a patient with SOX2 anophthalmia syndrome. Am J Med Genet A. 2010;152A:2355–9.
Oberoi S, Vargervik K. Hypoplasia and hypodontia in Van der Woude syndrome. Cleft Palate Craniofac J. 2005;42:459–66.
Ohazama A, Sharpe PT. TNF signalling in tooth development. Curr Opin Genet Dev. 2004;14:513–9.
Ohazama A, Hu Y, Schmidt-Ullrich R, Cao Y, Scheidereit C, Karin M, Sharpe. A dual role for Ikka in tooth development. Dev Cell. 2004;6:219–27.
Ohazama A, Sharpe PT. Development of epidermal appendages; teeth and hair. In: Epstein CJ, Erickson RP, Wynshaw-Boris A, editors. Inborn errors of development. The molecular basis of clinical disorders of morphogenesis. 2nd ed. Oxford: Oxford University Press; 2008. p. 245–62.
Ohazama A, Johnson EB, Ota MS, Choi HY, Porntaveetus T, Oommen S, Itoh N, Eto K, Gritli-Linde A, Herz J, Sharpe PT. Lrp4 modulates extracellular integration of cell signaling pathways in development. PLoS One. 2008;3, e4092.
Ohazama A, Haycraft CJ, Seppala M, Blackburn J, Ghafoor S, Cobourne M, Martinelli DC, Fan CM, Peterkova R, Lesot H, Yoder BK, Sharpe P. Primary cilia regulate Shh activity in the control of molar tooth number. Development. 2009;136:897–903.
Oommen S, Otsuka-Tanaka Y, Imam N, Kawasaki M, Kawasaki K, Jalani-Ghazani F, Anderegg A, Awatramani R, Hindges R, Sharpe PT, Ohazama A. Distinct roles of microRNAs in epithelium and mesenchyme during tooth development. Dev Dyn. 2012;241:1465–72.
Pawlowska E, Janik-Papis K, Poplawski T, Blasiak J, Szczepanska J. Mutations in the PAX9 gene in sporadic oligodontia. Orthod Craniofac Res. 2010;13:142–52.
Peterkova R, Lesot H, Peterka M. Phylogenetic memory of developing mammalian dentition. J Exp Zool B Mol Dev Evol. 2006;306:234–50.
Peters H, Neubuser A, Kratochwil K, Balling R. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 1998;12:2735–47.
Pirinen S, Kentala A, Nieminen P, Varilo T, Thesleff I, Arte S. Recessively inherited lower incisor hypodontia. J Med Genet. 2001;38:551–6.
Pispa J, Jung HS, Jernvall J, Kettunen P, Mustonen T, Tabata MJ, Kere J, Thesleff I. Cusp patterning defect in Tabby mouse teeth and its partial rescue by FGF. Dev Biol. 1999;216:521–34.
Plikus MV, Zeichner-David M, Mayer JA, Reyna J, Bringas P, Thewissen JG, Snead ML, Chai Y, Chuong CM. Morphoregulation of teeth: modulating the number, size, shape and differentiation by tuning Bmp activity. Evol Dev. 2005;7:440–57.
Rajab LD, Hamdan MA. Supernumerary teeth: review of the literature and a survey of 152 cases. Int J Paediatr Dent. 2002;12:244–54.
Ranta R. A review of tooth formation in children with cleft lip/palate. Am J Orthod Dentofacial Orthop. 1986;90:11–8.
Satokata I, Maas R. Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat Genet. 1994;6:348–56.
Schalk-van der Weide Y, Steen WH, Bosman F. Taurodontism and length of teeth in patients with oligodontia. J Oral Rehabil. 1993;20:401–12.
Schneider EL. Lip pits and congenital absence of second premolars: varied expression of the Lip Pits syndrome. J Med Genet. 1973;10:346–9.
Schwabe GC, Opitz C, Tinschert S, Mundlos S, Sharpe PT. Molecular mechanisms of tooth development and malformations. Oral Biosci Med. 2004;1:77–91.
Shapira Y, Lubit E, Kuftinec MM. Congenitally missing second premolars in cleft lip and cleft palate children. Am J Orthod Dentofacial Orthop. 1999;115:396–400.
Shapira Y, Kuftinec MM. Tooth transpositions – a review of the literature and treatment considerations. Angle Orthod. 1989;59:271–6.
Van Buggenhout G, Bailleul-Forestier I. Mesiodens. Eur J Med Genet. 2008;51:178–81.
van den Boogaard MJ, Créton M, Bronkhorst Y, van der Hout A, Hennekam E, Lindhout D, Cune M, Ploos van Amstel HK. Mutations in WNT10A are present in more than half of isolated hypodontia cases. J Med Genet. 2012;49:327–31.
van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R. Development of several organs that require inductive epithelial–mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8:2691–703.
Vastardis H, Karimbux N, Guthua SW, Seidman JG, Seidman CE. A human MSX1 homeodomain missense mutation causes selective tooth agenesis. Nat Genet. 1996;13:417–21.
Wang XP, Fan J. Molecular genetics of supernumerary tooth formation. Genesis. 2011;49:261–77.
Wang XP, O’Connell DJ, Lund JJ, Saadi I, Kuraguchi M, Turbe-Doan A, Cavallesco R, Kim H, Park PJ, Harada H, Kucherlapati R, Maas RL. Apc inhibition of Wnt signaling regulates supernumerary tooth formation during embryogenesis and throughout adulthood. Development. 2009;136:1939–49.
Wang XP, Suomalainen M, Felszeghy S, Zelarayan LC, Alonso MT, Plikus MV, Maas RL, Chuong CM, Schimmang T, Thesleff I. An integrated gene regulatory network controls stem cell proliferation in teeth. PLoS Biol. 2007;5:e159.
Yang Y, Luo L, Xu J, Zhu P, Xue W, Wang J, Li W, Wang M, Cheng K, Liu S, Tang Z, Ring BZ, Su L. Novel EDA p.Ile260Ser mutation linked to non-syndromic hypodontia. J Dent Res. 2013;92:500–6.
Yoshizaki K, Hu L, Nguyen T, Sakai K, He B, Fong C, Yamada Y, Bikle DD, Oda Y. Ablation of coactivator Med1 switches the cell fate of dental epithelia to that generating hair. PLoS One. 2014;9:e99991.
Zhang Z, Lan Y, Chai Y, Jiang R. Antagonistic actions of Msx1 and Osr2 pattern mammalian teeth into a single row. Science. 2009;323:1232–4.
Zhou P, Byrne C, Jacobs J, Fuchs E. Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes Dev. 1995;9:700–13.
Zhu JF, Marcushamer M, King DL, Henry RJ. Supernumerary and congenitally absent teeth: a literature review. J Clin Pediatr Dent. 1996;20:87–95.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
This chapter is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the work’s Creative Commons license, unless indicated otherwise in the credit line; if such material is not included in the work’s Creative Commons license and the respective action is not permitted by statutory regulation, users will need to obtain permission from the license holder to duplicate, adapt or reproduce the material.
Copyright information
© 2017 The Author(s)
About this paper
Cite this paper
Kawasaki, M., Kawasaki, K., Blackburn, J., Ohazama, A. (2017). Molecular Mechanisms Regulating Tooth Number. In: Sasaki, K., Suzuki, O., Takahashi, N. (eds) Interface Oral Health Science 2016. Springer, Singapore. https://doi.org/10.1007/978-981-10-1560-1_13
Download citation
DOI: https://doi.org/10.1007/978-981-10-1560-1_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-1559-5
Online ISBN: 978-981-10-1560-1
eBook Packages: MedicineMedicine (R0)