Abstract
Protein cavities or voids are observed as defects in atomic packing. Cavities have long been suggested to play important roles in protein dynamics and function, but the underlying origin and mechanism remains elusive. Here, recent studies about the cavities characterized by high-pressure NMR spectroscopy have been reviewed. Analysis of the pressure-dependent chemical shifts showed both linear and nonlinear response of proteins to pressure. The linear response corresponded to compression within the native ensemble, while the nonlinear response indicated the involvement of low-lying excited states that were different from the native state. The finding of non-linear pressure shifts in various proteins suggested that the existence of the low-lying excited states was common for globular proteins. However, the absolute nonlinear coefficient values varied significantly from protein to protein, and showed a good correlation with the density of cavities. Extensive studies on hen lysozyme as a model system showed that the cavity hydration and water penetration into the interior of proteins was an origin of the conformational transition to the excited states. The importance of cavities for protein function and evolution has also been explained. In addition to these “equilibrium” cavities, there are also “transient” cavities formed in the interior of the protein structure, as manifested by the ring flip motions of aromatic rings. The significance of transient cavities, reflecting an intrinsic dynamic nature within the native state, has also been discussed.
Similar content being viewed by others
Keywords
- Cavity
- Conformational fluctuation
- High-energy sub-states
- High-pressure NMR spectroscopy
- Hydration
- Low-lying excited states
1 Introduction
Protein cavities or voids are observed stably as “static” defects in atomic packing in crystal structures, but their occurrence and size vary from protein to protein. In addition to those “static” cavities, there are “transient” cavities formed also in the interior of the protein structure that are rather rarely detected in solution. The latter demonstrates directly the intrinsically dynamic nature of the folded protein structure in solution. On the other hand, the “static” cavities are not really static, but have been considered the source of dynamics for a long time based on the recognition of their contribution to the compressibility of protein molecules (Gekko et al. 1986). Later, high-pressure fluorescence study revealed their roles in pressure denaturation of proteins (Pin et al. 1990). In more recent years, high pressure NMR spectroscopy is beginning to reveal their roles in dynamics and high-energy sub-states in proteins. The manner how cavities provide sources for internal hydration, dynamics and high-energy sub-states is becoming increasingly clear, together with their roles in function. In this chapter, by reviewing some representative examples using pressure as a perturbation, we will discuss the effect of both “transient” and “static” cavities on protein dynamics, high-energy sub-states and function.
2 Cavities and Excited States in Proteins
Under physiological conditions, a protein molecule folds into a stable three-dimensional structure with close atomic packing. In the crystal structure, the folded structure of a protein is often encountered with a void or atomic defect where the density of atoms of the protein is extremely low. This is called a “static” cavity or, may be more correctly, an “equilibrium” cavity (See Fig. 5.2 in Chap. 5), as they are considered to exist in thermodynamic equilibrium in solution. There are two categories of the equilibrium cavities. One is a nonpolar (or hydrophobic) cavity that is surrounded by non-polar side chains. The other is a polar (or hydrophilic) cavity that is surrounded, at least in part, by polar side chains and/or main chain of peptide groups. In the latter case, water molecules inside the cavity are hydrogen bonded to polar groups of the main chain or side chains (Liu et al. 2008). In the former case, the question still remains whether nonpolar cavities in proteins are truly empty (Matthews and Liu 2009). In this Sect. 12.2, we describe the definition of the equilibrium cavities, high pressure NMR spectroscopy, and linearity analysis of the high pressure NMR data. This analysis clearly shows that the existence of low-lying excited states is usually hidden inside the large population of the native state.
2.1 Equilibrium Cavities Detected in Protein Structures
The intrinsic volume of a protein, which can be determined from its X-ray crystal structure, may be defined as the sum of van der Waals volumes of the constitutive atoms (v c), the volume of the cavities (v cav) and the volume change due to solvation or hydration (Δv sol) on protein surface:
(cf. Gekko, Chap. 5). The cavity is defined by rolling a probe of smaller radius (∼1.2 Å) inside the protein molecule. The internal cavity and solvent-accessible surface (SAS) are illustrated in Fig. 5.2. SAS is defined as a surface traced by the center of a spherical probe of appropriate radius (water molecule of 1.4 Å for most purposes) rolling on the molecular surface.
Several softwares and algorithms have been published for the calculation of cavities and/or SAS, such as VOIDOO (Kleywegt and Jones 1994), GRASP (Nicholls et al. 1991), VOLBL (Liang et al. 1998a, b), POCKET (Levitt and Banaszak 1992), and a Monte Carlo (MC) procedure (Chakravarty et al. 2002), etc. Recently, several web servers have also been developed to calculate protein cavities, such as 3 V server (Voss and Gerstein 2010) located at http://3vee.molmovdb.org, and CASTp (Computed Atlas of Surface Topography of proteins) server (Binkowski et al. 2003) located at http://sts.bioengr.uic.edu/castp/.
2.2 Pressure as a Tool for Investigating Cavity and Hydration of Proteins
Pressure is a fundamental thermodynamic variable for defining protein conformational states, and volume is the conjugate variable for pressure. The partial molar volume is composed of the volume of the protein constituent atoms, the cavity, and the hydration layer. Therefore, pressure is a unique and appropriate perturbation to study the cavity and its hydration of the protein, since the volume of the atoms does not change with pressure.
In the past, pressure and volume effects on the protein structure, dynamics, and function have been studied macroscopically by measuring compressibility (Gekko and Hasegawa 1986). More recently, with the advancement of high-pressure NMR technique (Akasaka and Yamada 2001), high-pressure macromolecular crystallography (HPMX) (Girard et al. 2005; Fourme et al. 2011), and molecular dynamics simulation at high pressure (Imai et al. 2007; Sgourakis et al. 2008; Imai and Sugita 2010), pressure is increasingly used to study structural fluctuations and transitions into alternate conformations in atomic level details. Among these, high-pressure NMR has been the most versatile technique to probe a wide range of conformational fluctuations of protein molecules in solution (Kamatari et al. 2004; Akasaka 2006; Li and Akasaka 2006).
2.3 Excited States in Proteins Revealed from Nonlinear Chemical Shifts
The chemical shift is a sensitive probe to study the pressure response of protein structure. Both amide 1H and 15N and 13C shifts reflect site-specific structural changes in the polypeptide backbone. The amide 1H pressure shift is particularly well correlated with the H…O hydrogen-bond (H-bond) distance (Li et al. 1998), whereas 15N and 13C pressure shifts are more sensitive to variations in the main chain torsion angles ϕ and ψ (Akasaka et al. 1999; Kalbitzer et al. 2000; Wilton et al. 2009). Therefore, the dependence of the 1H, 15N, and 13C chemical shifts on pressure would indicate the response of the H-bond distance and torsion angles to pressure.
2.3.1 Analysis of Pressure Response of Chemical Shifts
When performing high-pressure NMR measurements of proteins, we encountered cases showing linear and distinct nonlinear pressure shifts of amide 1H and 15N signals in many proteins (Fig. 12.1a–c), and even sigmoidal curves (Fig. 12.1d–e). Then we performed the estimation of linearity and nonlinearity of chemical shift changes with pressure (Akasaka and Li 2001; Kitahara et al. 2013), which is derived from least-square fits of experimental data for individual 1H and 15N signals to the following equation:
where P is the pressure (bars), δ i is the chemical shift (parts per million) for the ith residue, a i (parts per million) is the chemical shift at 1 bar, and b i (parts per million per bar) and c i (parts per million per squared bar) are the linear (first-order) and nonlinear (second-order) coefficients, respectively.
Nature of the linear and nonlinear pressure response. Plot of chemical shifts of selected amide protons of (a) BPTI, (b) DHFR, and (c) RalGDS-RBD as a function of pressure. Pressure dependence of (d) 15N and (e) 1H chemical shifts of Val70 of ubiquitin at 20 °C (Adapted from Fig. 1 in Akasaka and Li (2001) and Fig. 6 in Kitahara et al. (2001), respectively, by permission of American Chemical Society)
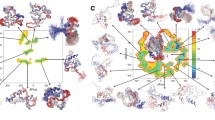
In Fig. 12.2, the correlation plots of the linear (vertical axis) and nonlinear (horizontal axis) coefficients of amide 15N are shown for 16 proteins with different structures. As clearly seen in Fig. 12.2, the distribution of the nonlinear coefficients varies from protein to protein, while that of the linear coefficients is similar for all proteins. Therefore, the quantitative analysis of the linear and nonlinear coefficients was performed for each protein.
Correlations between the linearity and nonlinearity coefficients of pressure-induced 15N shifts. In each figure, the linear coefficient is plotted against the non-linear coefficient for individual residues of each protein: (a) protein G, (b) BPTI, (c) ubiquitin, (d) NEDD8, (e) parkin-UBL, (f) HPr (WT), (g) HPr (H15A), (h) RasRBD, (i) RasRBD complex, (j) p13, (k) α-lactalbumin (MG), (l) lysozyme, (m) prion protein, (n) apomyoglobin, (o) β-lactoglobulin, (p) OspA. α-helix, β-sheet, and other regions are colored by red, blue, and black, respectively (Adapted from Fig. 17 in Kitahara et al. (2013) by permission of Elsevier Inc)
As shown in Fig. 12.3a (dotted columns), the average value of linear coefficients varies little for the proteins investigated. On the other hand, the degree of nonlinearity varies greatly from protein to protein and appears to be characteristic for each protein (Fig. 12.3a, filled columns).
(a) Histograms of the mean of the absolute values of the first-order coefficient b i (dotted columns) and the second-order coefficient c i (filled columns) for 1HN in eight globular proteins. (b) Plot of the mean of the absolute values of the second-order coefficient c i of 1HN pressure shifts vs the density of cavities (the total cavity volume divided by the number of amino acid residues) for the eight proteins. Cavity volumes of >20 Å3 are employed (calculated by GRASP using PDB coordinates) (Adapted from Fig. 3 in Akasaka and Li (2001) by permission of American Chemical Society)
2.3.2 Nature of the Linear and Nonlinear Pressure Response
The linear chemical-shift change with pressure results from a linear change in the averaged inter-nuclear distances and torsion angles, the combination of which determines the compressibility coefficient (β T) of the protein. The observation of a linear pressure shift, therefore, indicates that β T is independent of pressure within the pressure range studied (approximately 0.1–200 MPa) and arises from a small shift of population within the basic folded ensemble. The similarity of linear coefficients among proteins would mean that the amplitude of structural fluctuation (inter-nuclear distances and torsion angles of the main chain) is of a similar magnitude for all these proteins at a pressure of 0.1 MPa, namely in the ground state of the protein conformation.
On the other hand, the remarkable nonlinearity of pressure shifts is explained by the involvement of an ensemble N′ different from the basic folded ensemble N. N′ not only has a compressibility different from N, giving different slopes of pressure shifts, but also has a partial molar volume smaller than that of N so that its relative population ([N′]/[N]) increases with increasing pressure. The distinct nonlinear pressure shifts over a relatively low-pressure range (approximately 50–100 MPa) indicate that the population of N′ increases significantly with pressure, even at relatively low pressure. This means that the Gibbs-free energy difference between N and N′ (ΔG 0) is not very large (of the order of a few kJ mol−1), or in other words, that N′ is a low-lying excited state of a protein. Furthermore, in general, it is likely that N′ involves a family of different conformers (such as N′, N″, N‴, etc., evidenced in Sect. 12.3.2 later), or subensembles, with different ΔG 0 and ΔV 0 values. The finding of non-linear pressure shifts in various proteins suggests that low-lying excited states are common in many globular proteins, but that their fractional populations vary significantly from protein to protein.
2.3.3 Structure of the Excited States
As an ideal nonlinear response, sigmoidal changes in pressure-induced chemical shifts have been observed in ubiquitin in the pressure range from 3 to 350 MPa at 20 °C (Kitahara et al. 2001). This is a clear evidence for a two-state like transition between the basic folded state (N) and a low-lying excited state (N′). In this case, the detailed structural analysis of an excited-state conformer is even possible using NMR spectroscopy, which was manifested by the first excited-state structure of ubiquitin at 300 MPa (Kitahara et al. 2005). Kitahara et al. found that the major difference between N and N′ of ubiquitin is that N′ has an open structure in its central cleft.
2.3.4 Origin of Nonlinearity and Excited States
The population of N′ increases with pressure implies that the N′ state has a lower partial volume than the N state, suggesting that the N′ state is likely to be more hydrated than N. In support of this view, a nonlinear shift is observed for residues close to water-accessible cavities within the folded protein structure in BPTI (Williamson et al. 2003), hen lysozyme (Kamatari et al. 2001), RalGDS-RBD (Inoue et al. 2000), and β-lactoglobulin (Kuwata et al. 2001). Furthermore, a good correlation is found between the mean of the absolute nonlinear coefficient values for amide protons and the density of cavities (the total volume of cavities divided by the number of amino acid residues) in the native ensemble of these proteins (Fig. 12.3b). These findings suggest that the nonlinear shift is related to the hydration of water-accessible cavities. Indeed, an X-ray crystallography study on the L99A cavity mutant of T4 lysozyme showed an increase in the number of internal water molecules within the created cavity at higher pressures (Collins et al. 2005).
An intriguing finding from an exceptionally long (μs) molecular dynamics simulation carried out for ubiquitin at 1 bar and 6,000 bar by Imai and Sugita is that the larger amplitude transition occurs simultaneously with the penetration of water molecules into the central cleft of the ubiquitin (Imai and Sugita 2010). This MD result is consistent with an experimental observation about the volume loss from N to N′ (ΔV = −24 mL mol−1) (Kitahara et al. 2005). In the conformer N′, most of the residues involved in the enzyme-binding are exposed. Thus, it is likely that the low-lying excited state does not correspond to a single structure but spans a continuous range of structures with different hydration numbers and locations (Kamatari et al. 2011). When the hydration becomes substantial, it will collapse into local or total unfolding, making partial or the entire polypeptide segment hydrated and exposed, as is the case for RalGDS-RBD (Inoue et al. 2000), ubiquitin (Kitahara et al. 2005), and β-lactoglobulin (Kuwata et al. 2001).
2.3.5 Timescale of the Equilibrium of Excited States
N and N′ states exist in equilibrium and undergo conformational exchange. The continuous chemical shift changes upon pressure suggest that the equilibrium between N and N′ is fast within the NMR timescale, i.e. faster than μs (or 106 s−1). At even higher pressure, an ideal nonlinear response, i.e. sigmoidal changes, in pressure-induced chemical shifts would be observed for some proteins. This is a clear evidence for a two-state like transition between the basic folded state (N) and a low-lying excited state (N′), as illustrated above for ubiquitin in the pressure range from 3 to 350 MPa at 20 °C (Kitahara et al. 2001). Kitahara et al. further found that N and N′ are in dynamic equilibrium, and the exchange rate between the two states are on the timescale of ∼10 μs (or ∼105 s−1). Furthermore, depending on the protein, when the equilibrium between N and N′ is slow with respect to chemical shift differences (on the timescale of 104 s−1 or even slower), instead of the continuous chemical shift changes, peak intensity decrease of N will be observed, implying the existence of an excited state with higher energy, or an intermediate state.
2.3.6 Comparison with Temperature Response of Chemical Shifts
Williamson group had analyzed the curvature of temperature dependences of amide proton NMR chemical shifts of proteins before (Baxter et al. 1998) and demonstrated the existence of the low free-energy excited states in folded proteins. They found that the source of conformational heterogeneity includes many independent causes, such as the existence of a minor conformer, an alternative hydrogen bond network of the amide proton involved, etc. Although the factor of cavity is not included in their analysis, the importance of buried waters is also demonstrated from the temperature response.
3 Water Penetration into Cavities: Hen Lysozyme
In this section, we use hen lysozyme as a model system to show the significant role of the cavities played in protein dynamics, structural transition, protein function, and evolution.
3.1 Compression and Mobility in the Folded Hen Lysozyme
Kamatari et al. used high-pressure NMR techniques to characterize the conformational fluctuations of hen lysozyme, in its native state and when denatured in 8 M urea, over the pressure range 30–2,000 bar (Kamatari et al. 2001). All the NMR signals showed continuous and fully reversible changes in chemical shifts with no sign of pressure denaturation in this pressure range (Fig. 12.4a). Significant variations in pressure shifts from their average values are observed for both 1H and 15N in native lysozyme (Fig. 12.4a, c and d). Especially, some residues in the β domain or at the α/β domain interface have anomalously large 15N and 1H chemical-shift changes. All these residues lie close to water-containing cavities (Fig. 12.5), suggesting that the cavities are the source of conformational changes. In contrast, the pressure-induced 1H and 15N shifts for lysozyme denatured in 8 M urea, which is unfolded and has no cavity, are much more uniform than those for the folded lysozyme (Fig. 12.4b, e and f). All these results suggest that the anomalous pressure shifts of amide 1H and 15N signals in the state arise mainly from structural rearrangements of these cavities, including the buried water molecules inside, with increasing pressure. Therefore, cavities provide a source of compression and mobility in the folded lysozyme.
Overlay of 15N-1H HSQC spectra of (a) native hen lysozyme and (b) hen lysozyme denatured in 8 M urea at pH 2 recorded at high pressure. Cross-peaks from spectra recorded at 30, 500, 1,000, 1,500 and 2,000 bar. The folded cross-peaks are shown enclosed in boxes in the spectra. 15N and 1H pressure shifts (ΔδN and ΔδH, respectively) for the main-chain amide groups of native (c and d) and denatured (e and f) hen lysozyme [Δδ = δ (2,000 bar) − δ (30 bar)]. The positions in the protein sequence of the α helices and the triple-stranded antiparallel β sheet in native lysozyme are indicated above the diagram. (Adapted from Fig. 1 and 3 in Kamatari et al. (2001) by permission of John Wiley & Sons, Inc)
Compression and mobility in the native hen lysozyme. Main-chain trace of native hen lysozyme (structure 2lzt) showing the positions of the six buried water molecules (indicated by blue spheres) in cavities within the structure. The α domain of the protein (residues 1–35, 85–129) is shown in light grey and the β domain (residues 36–94) in dark grey. Residues with large main chain 15N pressure shifts (>1.3 or < 0.20 ppm) are shown in red (Tyr53, Ile55, Ser60, Arg73, Ser91), those with large 1H pressure shifts (>0.25 or < −0.08 ppm) in green (Trp62, Trp63, Asn65, Asp66, Gly67), and that with large 15N and 1H pressure shifts in yellow (Asn74) (Adapted from Fig. 5 in Kamatari et al. (2001) by permission of John Wiley & Sons, Inc)
Williamson and co-workers have calculated the protein structure under high-pressure using the pressure-induced 1H chemical shift changes as restraints (Refaee et al. 2003). The structural changes of hen lysozyme with pressure calculated by them are shown in Fig. 6.2 in Chap. 6. An interesting finding is that, consistent with the above pressure response of chemical shifts, the largest volume changes tend to occur close to water-containing cavities.
3.2 Cavity Hydration as a Gateway to Unfolding
We have been focusing on conformational fluctuations localized around water contained in cavities within the ensemble of native conformers of hen lysozyme. The next question is how the initial step of the conformational transition would be caused from the native state to excited states of this protein. For this purpose, Kamatari et al. used low temperatures (down to −20 °C) and high pressures (up to 2,000 bar) to populate low-lying excited state conformers of hen lysozyme, and analyzed their structures site-specifically using 1H one-dimensional and 15N/1H two-dimensional HSQC NMR spectroscopy (Fig. 12.6). The experiment condition is taking the advantage of the fact that the freezing point of water is decreased to −21 °C at 2,000 bar (Jonas 1982). As shown in the 1H one-dimensional spectra (Fig. 12.6a), the signals gradually broaden as the temperature is lowered, although the chemical shifts are largely unchanged even at the lowest temperature, −20 °C, reached at 2,000 bar, revealing that the overall structure of the native state is retained down to −20 °C at 2,000 bar. However, the features of the 15N/1H HSQC spectra recorded over the corresponding temperature range show very different behavior (Fig. 12.6b), the cross-peaks being selectively broadened above −10 °C and almost completely disappearing below −15 °C. The resulting disappearance of cross-peaks includes those of residues in the α-domain of the protein and the cleft between the α- and β-domains, both located close to water-containing cavities (Fig. 12.7a). The disappearance of some cross-peaks is often accompanied by the appearance of new peaks, indicating that a segment of the protein is denatured. However, this is not the case. Instead, the data shown in Fig. 12.6 revealed that the α-domain is not yet fully denatured but is likely to be partially disordered and heterogeneous in conformation around the original natively folded structure N. The high degree of broadening can be attributed to exchange effect that simply requires an increased dispersion of 1H and 15N chemical shifts. To cause the broadening of the NMR signals, the rate of exchange among these heterogeneous conformations must be rather slow on the NMR time scale of ∼μs∼ms (or ∼103 ∼ 106 s−1). The ensemble of conformers with substantial heterogeneity in the β-domain can be attributed to an ensemble of low-lying excited state conformers (N′, N″, N‴, etc.) stabilized under the condition of low temperature and high pressure relative to the stable folded conformer N (Fig. 12.7b). The fact that their populations are increased with increasing pressure indicates that they have slightly smaller partial molar volumes than the conformer N, consistent with a view that they are more hydrated than the N conformer.
Changes in NMR spectra of hen lysozyme at high pressure and low temperature. (a) 1H one-dimensional NMR spectra of 15N-uniformly labeled hen lysozyme (1.7 mM, pH 3.8) at 2,000 bar and various temperatures indicated. (b) 15N/1H two-dimensional HSQC spectra at 2,000 bar and various temperatures. Cross-peaks that disappear between 15 °C and 10 °C are labeled on the spectrum recorded at 10 °C and that disappear between 10 °C and 5 °C are labeled on the spectrum at 5 °C (Adapted from Fig. 1 in Kamatari et al. (2011) by permission of Elsevier Inc)
NMR images of native hen lysozyme. (a) From 15N/1H HSQC cross-peak intensities at low temperature and 2,000 bar. The residues whose main-chain amide resonances disappear from the HSQC spectrum at temperatures above 7.5 °C, 2.5 °C, and 0 °C are shown in red, orange, and yellow, respectively. The main-chain trace of native hen lysozyme (structure 2lzt) showing the positions of the six buried water molecules (indicated by blue spheres) in cavities within the structure. (b) Probable locations of low-lying excited states (N′, N″, N‴, …) and α-domain intermediate depicted in this study on the schematic free-energy (F) surface representing features of the folding of hen lysozyme. Qα and Qβ are the numbers of contacts in the α-and β-domains. The right trajectory represents a “fast track” in which α/β intermediates are populated only transiently. The left trajectory represents a “slow track” in which both α/β and α intermediates are populated transiently, depending on the time-scale (Adapted from Fig. 3 and 4 in Kamatari et al. (2011) by permission of Elsevier Inc)
In our previous 15N/1H HSQC NMR study of hen lysozyme carried out at the conditions under which the folded conformer is quite stable, we showed from pressure-induced chemical shifts that the fluctuations of the folded structure are most evident in the vicinity of the water-containing cavities (Fig. 12.5). If we compare Figs. 12.5 and 12.7a, residues in the vicinity of the water-containing cavities are commonly perturbed. Then is there any correlation between the two dynamic processes, i.e., conformational fluctuations within the ensemble of folded conformations and conformational transitions to low-lying excited states? The finding suggests that the conformational fluctuations within the folded manifold are localized to the vicinity of the cavities but give rise to significant heterogeneity and disorder in the conformational ensemble involving a large number of residues in the α-domain.
Furthermore, 15N/1H HSQC NMR study of hen lysozyme carried out at higher pressure and lower temperature shown here provides an example to link the equilibrium intermediate stabilized by pressure and the kinetic intermediate in folding, the two conformers being generally coincident (Kitahara et al. 2003). Therefore, high-pressure NMR can provide a unique opportunity for studying structural details of kinetic intermediates involved in protein folding. In lysozyme, the preferential disappearance of signals from the entire β-domain seems to represent the hydration event just prior to the complete solvation or denaturation of the β-domain, leading to the formation of the “α-domain intermediate”, found in the kinetic experiments (Radford et al. 1992; Miranker et al. 1993). Probable locations of these low-lying excited states and the α-domain intermediate on the free-energy surface are shown in Fig. 12.7b. Thus the study supports the view that cavities are the source of water penetration into the protein interior and lead to the formation of low-lying excited states.
3.3 Water Penetration into Hen Lysozyme Interior: MD Simulation Study
Recently, Soda et al. presented a new method for determining the hydration site of proteins using MD simulation, where the effect of structural fluctuations and hydration water was explicitly considered (Soda et al. 2011). Probability distribution of the total number of internal hydration waters and the spatial distribution of the internal hydration waters in the interior of hen lysozyme is shown in Fig. 12.8. It is interesting to find that internal hydration waters distribute fairly widely over the whole protein as well as in the major internal hydration sites of the hinge region (center) and the loop region in the β-domain (left side). These regions are just the places where the non-linear pressure shifts are distributed from the high-pressure NMR study (Kamatari et al. 2001).
(a) Probability distribution of the total number of internal hydration waters for the whole hydration sites, with its mean of 4.81. (b) Distribution of internal hydrated waters in the interior of hen lysozyme. The internal hydrated waters distribute fairly widely over the whole protein as well as in the major internal hydration sites of the hinge region (center) and the loop region in the β-domain (left side) (Adapted from Fig. 2 in Soda et al. (2011) by permission of Elsevier Inc)
3.4 Cavity is Evolutionarily Conserved for Protein Function
As mentioned in the previous section, cavity and hydration play important roles for protein dynamics. Therefore, cavity and hydration also expected to play important roles for protein function. Here, we show an example related to cavity and function. Locations of water-containing cavities in the crystal structures of lysozymes from a variety of species are shown in Fig. 12.9 (Kamatari et al. 2011). The figure shows that the locations of the water-containing cavities are remarkably similar among the lysozyme molecules from different biological origin, despite the fact that the amino acid sequences are not highly conserved (for example, the sequence identity of hen and dog lysozyme is less than 50 %). This observation suggests that the cavities could be an important feature of the lysozyme function, and is consistent with the view that the cavities play a crucial role in the catalytic cycle of lysozymes by giving a degree of mobility in the active site, always with a well-defined population of water molecules that could contribute to the hydrolysis of the substrate molecules. This result also suggests cavities are evolutionarily conserved for protein function.
Locations of cavities and buried water molecules (indicated by blue spheres) in a variety of structures of lysozyme from different biological species: hen, bobwhite, quail, turkey, trout, human, echidna, and dog (Adapted from Fig. 5 in Kamatari et al. (2011) by permission of Elsevier Inc)
4 Transient Cavities and Protein Dynamics
All cavities described above in this chapter exist in equilibrium. However, cavity can also appear transiently by the conformational fluctuations of proteins. One example is flip-flop motions of aromatic rings (Fig. 12.10a). In spite of close packing of amino acid residues in globular proteins, aromatic side chains buried in the protein interior undergo rotational motion. This phenomenon indicates that protein flexibility provides the necessary space for a ring to flip. The flip-flop motions reflect the dynamic feature of a protein, and the flip rates of the side chains serve as probes reporting the fluctuation of the interior of the folded structure.
Transient void detected through ring flip motions. (a) Illustration of the aromatic ring flip motion taking Phe as an example. The aromatic ring undergoes 180° flip motion along its Cβ–C1 axis. Right panel is the view from along Cβ–C1 axis. The dotted circles represent the atoms around Phe schematically. Flip-flop motion of Phe would result in the formation of transient cavities. (b) A ribbon model for BPTI with locations of Tyr35 and Phe45. (c) Superposition of the 3H and 5H region of the TOCSY slice spectra taken at 2H resonance position of Tyr35 measured at 57 °C at five different pressures. (d) Superposition of the 3H and 5H region of the TOCSY slice spectra taken at 2H resonance position of Phe45 measured at 57 °C at five different pressures. In (c) and (d), the arrow shows the direction of pressure increase: 1 bar (line), 500 bar (dotted line), 1,000 bar (dashed line), 1,500 bar (thick dotted line), and 2,000 bar (thick line). (e) Logarithmic plot of the flip-flop rate of the ring, k (in s−1), for Tyr35 and Phe45 against pressure (Adapted from Fig. 5 in Li et al. (1999) by permission of Elsevier Inc)
Temperature and pressure dependence of the flip rate is expected to bring crucial information on energy and volume fluctuations in the interior of the folded protein. The temperature dependence of the flip rate derives the activation enthalpy (ΔH ‡), while the pressure dependence of the flip rate derives the activation volume (ΔV ‡). Li et al. (1999) investigated the pressure-dependent ring flip motions of BPTI at 57 °C over the pressure range 30–2,000 bar using the 2D 1H NMR spectroscopy. The flip rate of each residue is estimated by the line shape simulation, and the activation volume is calculated from the pressure-dependent flip rate. As a result, the flip rate of each residue is estimated to be on the ms timescale, ranging from 10 s−1 to 104 s−1, and is decreased with the increase of pressure. Furthermore, the slope of the plot of the logarithms of the flip rates obtained from the simulation against pressure gives the ΔV ‡ value through the relation:
where k B is the Boltzmann constant, T is the absolute temperature, and k is the flip rate. The experimental results and data analysis of BPTI are shown in Fig. 12.10c–e. The ΔV ‡ value is defined as a difference between the volume occupied by the ring in the ground state and the volume in the activated state. The positive ΔV ‡ value shown in Table 12.1 indicates that the flip requires opening of the space surrounding the aromatic ring at the activation state. It is noteworthy that, despite the variety of microenvironments and types of the individual aromatic rings, the ΔV ‡ value is comparable to each other, ranging from 25 to 51 mL mol−1. This observation fits well with the notion that the flip requires a minimum common space or cavity to be created around the ring. This extra space must be created as a result of cooperative thermal fluctuations of atoms surrounding the aromatic ring, occurring infrequently (103–105 s−1) around it at ambient pressure. This means that a cavity is infrequently created in the most buried part of the protein (the core).
The first and the best-characterized case for the slowly exchanging aromatic residues is found in BPTI. Temperature and pressure dependence of the ring flip motions were investigated using 1D 1H NMR spectroscopy (Wagner et al. 1976, 1980, 1983). Li et al. renewed the pressure dependence the ring flip motions using 2D 1H NMR spectroscopy. Besides BPTI, temperature dependence of the rate of ring flip has been reported for phenylalanine and tyrosine residues of cytochrome c (Campbell et al. 1976) and hen lysozyme (Campbell et al. 1975). Later, pressure dependence of the rate of ring flip has been reported for HPr using high-pressure NMR techniques (Hattori et al. 2004). Recently, 13C transverse relaxation dispersion methods have been developed to characterize the ring-flip motion and renewed the rank order of ring-flip rates in BPTI (Weininger et al. 2014). In Table 12.1, the thermodynamic parameters for aromatic residues in BPTI, HPr, and cytochrome c are summarized.
It would be interesting to compare the activation volume with the equilibrium volume fluctuation of the protein. The root-mean-square fluctuation would be 0.3 ± 0.1 % of the total volume of the protein (Gekko and Hasegawa 1986). In the case of BPTI, the root-mean-square fluctuation is estimated to be only ∼24 Å3. Thus the local volume fluctuation, reflected by the activation volume, is far exceeding the fluctuation of the entire protein molecule. This result suggests that the formation of a large local cavity may be a consequence of accumulation of smaller cavities, and appears to be consistent with the dynamic nature of cavity formation (Kocher et al. 1996) or a mobile defect model such that water occasionally penetrates into the core of the protein (Lumry and Rosenberg 1975).
It is also interesting to notice that the activation volume of the ring-flip motions gives a positive value, while the volume change induced by pressure is negative. The positive value of the activation volume indicates transient opening of the space surrounding the aromatic ring at the activation state, reflecting the intrinsic dynamic nature of the N state. On the other hand, the negative volume change induced by pressure is caused by decrease of the cavity size or even water penetration into the cavities. The critical fluctuations of protein molecules, as manifested by ring-flips occurring in ms range, take place in a range far from the range of equilibrium fluctuation. As discussed in Sect. 12.2.3.5, however, the equilibrium of the low-lying excited states is in μs-ms range.
5 Summary
In this chapter, we have highlighted the importance of cavities for protein dynamics, stability, and function. The equilibrium cavities, caused by atomic packing defects, are commonly found inside protein molecules, and sometimes filled with internal waters molecules. Using MD simulation and high-pressure NMR spectroscopy, it has been shown that the equilibrium cavities provide the structural fluctuation, serve as sources of water penetration and hence the creation of low-lying excited states and even the gateway to unfolding. The analysis of the nonlinear chemical shifts induced by pressure has revealed the existence of the low-lying excited states in many globular proteins, while the equilibrium between the ground state and the excited states may be fast or slow. Furthermore, in the N state, besides the equilibrium cavities, the transient cavities have also been detected, as manifested by the ring-flip motions. The transient cavities accompanied by transient space opening, are intrinsic dynamic nature of the N state. Therefore, cavities, both equilibrium and transient, serve as the source of protein dynamics, and the evolutionarily conserved features of cavities and low-lying excited states of proteins are expected to shed new light on the molecular dynamism of proteins.
References
Akasaka K (2006) Probing conformational fluctuation of proteins by pressure perturbation. Chem Rev 106:1814–1835
Akasaka K, Li H (2001) Low-lying excited states of proteins revealed from nonlinear pressure shifts in 1H and 15N NMR. Biochemistry 40:8665–8671
Akasaka K, Yamada H (2001) On-line cell high-pressure nuclear magnetic resonance technique: application to protein studies. Methods Enzymol 338:134–158
Akasaka K, Li H, Yamada H, Li R, Thoresen T, Woodward CK (1999) Pressure response of protein backbone structure. Pressure-induced amide 15N chemical shifts in BPTI. Protein Sci 8:1946–1953
Baxter NJ, Hosszu LL, Waltho JP, Williamson MP (1998) Characterisation of low free-energy excited states of folded proteins. J Mol Biol 284:1625–1639
Binkowski TA, Naghibzadeh S, Liang J (2003) CASTp: computed atlas of surface topography of proteins. Nucleic Acids Res 31:3352–3355
Campbell ID, Dobson CM, Williams RJ (1975) Proton magnetic resonance studies of the tyrosine residues of hen lysozyme-assignment and detection of conformational mobility. Proc R Soc Lond B Biol Sci 189:503–509
Campbell ID, Dobson CM, Moore GR, Perkins SJ, Williams RJP (1976) Temperature dependent molecular motion of a tyrosine residue of ferrocytochrome C. FEBS Lett 70:96–100
Chakravarty S, Bhinge A, Varadarajan R (2002) A procedure for detection and quantitation of cavity volumes proteins. Application to measure the strength of the hydrophobic driving force in protein folding. J Biol Chem 277:31345–31353
Collins MD, Hummer G, Quillin ML, Matthews BW, Gruner SM (2005) Cooperative water filling of a nonpolar protein cavity observed by high-pressure crystallography and simulation. Proc Natl Acad Sci U S A 102:16668–16671
Fourme R, Girard E, Dhaussy AC, Medjoubi K, Prangé T, Ascone I, Mezouar M, Kahn R (2011) A new paradigm for macromolecular crystallography beamlines derived from high-pressure methodology and results. J Synchrotron Radiat 18:31–36
Gekko K, Hasegawa Y (1986) Compressibility-structure relationship of globular proteins. Biochemistry 25:6563–6571
Girard E, Kahn R, Mezouar M, Dhaussy AC, Lin T, Johnson JE, Fourme R (2005) The first crystal structure of a macromolecular assembly under high pressure: CpMV at 330 MPa. Biophys J 88:3562–3571
Hattori M, Li H, Yamada H, Akasaka K, Hengstenberg W, Gronwald W, Kalbitzer HR (2004) Infrequent cavity-forming fluctuations in HPr from Staphylococcus carnosus revealed by pressure- and temperature-dependent tyrosine ring flips. Protein Sci 13:3104–3114
Imai T, Sugita Y (2010) Dynamic correlation between pressure-induced protein structural transition and water penetration. J Phys Chem B 114:2281–2286
Imai T, Hiraoka R, Kovalenko A, Hirata F (2007) Locating missing water molecules in protein cavities by the three-dimensional reference interaction site model theory of molecular solvation. Proteins 66:804–813
Inoue K, Yamada H, Akasaka K, Herrmann C, Kremer W, Maurer T, Döker R, Kalbitzer HR (2000) Pressure-induced local unfolding of the Ras binding domain of RalGDS. Nat Struct Biol 7:547–550
Jonas J (1982) Nuclear magnetic resonance at high pressure. Science 216:1179–1184
Kalbitzer HR, Görler A, Li H, Dubovskii PV, Hengstenberg W, Kowolik C, Yamada H, Akasaka K (2000) 15N and 1H NMR study of histidine containing protein (HPr) from Staphylococcus carnosus at high pressure. Protein Sci 9:693–703
Kamatari YO, Yamada H, Akasaka K, Jones JA, Dobson CM, Smith LJ (2001) Response of native and denatured hen lysozyme to high pressure studied by 15N/1H NMR spectroscopy. Eur J Biochem 268:1782–1793
Kamatari YO, Kitahara R, Yamada H, Yokoyama S, Akasaka K (2004) High-pressure NMR spectroscopy for characterizing folding intermediates and denatured states of proteins. Methods 34:133–143
Kamatari YO, Smith LJ, Dobson CM, Akasaka K (2011) Cavity hydration as a gateway to unfolding: an NMR study of hen lysozyme at high pressure and low temperature. Biophys Chem 156:24–30
Kitahara R, Akasaka K (2003) Close identity of a pressure-stabilized intermediate with a kinetic intermediate in protein folding. Proc Natl Acad Sci U S A 100:3167–3172
Kitahara R, Yamada H, Akasaka K (2001) Two folded conformers of ubiquitin revealed by high-pressure NMR. Biochemistry 40:13556–13563
Kitahara R, Yokoyama S, Akasaka K (2005) NMR snapshots of a fluctuating protein structure: ubiquitin at 30 bar–3 kbar. J Mol Biol 347:277–285
Kitahara R, Hata K, Li H, Williamson MP, Akasaka K (2013) Pressure-induced chemical shifts as probes for conformational fluctuations in proteins. Prog Nucl Magn Reson Spectrosc 71:35–58
Kleywegt GJ, Jones TA (1994) Detection, delineation, measurement and display of cavities in macromolecular structures. Acta Crystallogr D Biol Crystallogr 50:178–185
Kocher JP, Prevost M, Wodak SJ, Lee B (1996) Properties of the protein matrix revealed by the free energy of cavity formation. Structure 4:1517–1529
Kuwata K, Li H, Yamada H, Batt CA, Goto Y, Akasaka K (2001) High pressure NMR reveals a variety of fluctuating conformers in β-lactoglobulin. J Mol Biol 305:1073–1083
Levitt DG, Banaszak LJ (1992) POCKET: a computer graphics method for identifying and displaying protein cavities and their surrounding amino acids. J Mol Graph 10:229–234
Li H, Akasaka K (2006) Conformational fluctuations of proteins revealed by variable pressure NMR. Biochim Biophys Acta 1764:331–345
Li H, Yamada H, Akasaka K (1998) Effect of pressure on individual hydrogen bonds in proteins. Basic pancreatic trypsin inhibitor. Biochemistry 37:1167–1173
Li H, Yamada H, Akasaka K (1999) Effect of pressure on the tertiary structure and dynamics of folded basic pancreatic trypsin inhibitor. Biophys J 77:2801–2812
Liang J, Edelsbrunner H, Fu P, Sudhakar PV, Subramaniam S (1998a) Analytical shape computation of macromolecules: I. Molecular area and volume through alpha shape. Proteins 33:1–17
Liang J, Edelsbrunner H, Woodward C (1998b) Anatomy of protein pockets and cavities: measurement of binding site geometry and implications for ligand design. Protein Sci 7:1884–1897
Liu L, Quillin ML, Matthews BW (2008) Use of experimental crystallographic phases to examine the hydration of polar and nonpolar cavities in T4 lysozyme. Proc Natl Acad Sci U S A 105:14406–14411
Lumry R, Rosenberg A (1975) The mobile defect hypothesis of protein function. Coll Int CNRS L’Eau Syst Biol 246:55–63
Matthews BW, Liu L (2009) A review about nothing: are apolar cavities in proteins really empty? Protein Sci 18:494–502
Miranker A, Robinson CV, Radford SE, Aplin RT, Dobson CM (1993) Detection of transient protein folding populations by mass spectrometry. Science 262:896–900
Nicholls A, Sharp KA, Honig B (1991) Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins 11:281–296
Otting G, Liepinsh E, Wüthrich K (1993) Disulfide bond isomerization in BPTI and BPTI(G36S): an NMR study of correlated mobility in proteins. Biochemistry 32:3571–3582
Pin S, Royer CA, Gratton E, Alpert B, Weber G (1990) Subunit interactions in hemoglobin probed by fluorescence and high-pressure techniques. Biochemistry 29:9194–9202
Radford SE, Dobson CM, Evans PA (1992) The folding of hen lysozyme involves partially structured intermediates and multiple pathways. Nature 358:302–307
Refaee M, Tezuka T, Akasaka K, Williamson MP (2003) Pressure-dependent changes in the solution structure of hen egg-white lysozyme. J Mol Biol 327:857–865
Sgourakis NG, Day R, McCallum SA, Garcia AE (2008) Pressure effects on the ensemble dynamics of ubiquitin inspected with molecular dynamics simulations and isotropic reorientational eigenmode dynamics. Biophys J 95:3943–3955
Soda K, Shimbo Y, Seki Y, Taiji M (2011) Structural characteristics of hydration sites in lysozyme. Biophys Chem 156:31–42
Voss NR, Gerstein M (2010) 3V: cavity, channel and cleft volume calculator and extractor. Nucleic Acids Res 38:W555–W562
Wagner G (1980) Activation volumes for the rotational motion of interior aromatic rings in globular proteins determined by high resolution 1H NMR at variable pressure. FEBS Lett 112:280–284
Wagner G (1983) Characterization of the distribution of internal motions in the basic pancreatic trypsin inhibitor using a large number of internal NMR probes. Q Rev Biophys 16:1–57
Wagner G, DeMarco A, Wüthrich K (1976) Dynamics of the aromatic amino acid residues in the globular conformation of the basic pancreatic trypsin inhibitor (BPTI). I. 1H NMR studies. Biophys Struct Mech 2:139–158
Weininger U, Modig K, Akke M (2014) Ring flips revisited: 13C relaxation dispersion measurements of aromatic side chain dynamics and activation barriers in basic pancreatic trypsin inhibitor. Biochemistry 53:4519–4525
Williamson MP, Akasaka K, Refaee M (2003) The solution structure of bovine pancreatic trypsin inhibitor at high pressure. Protein Sci 12:1971–1979
Wilton DJ, Kitahara R, Akasaka K, Williamson MP (2009) Pressure-dependent 13C chemical shifts in proteins: origins and applications. J Biomol NMR 44:25–33
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Li, H., Kamatari, Y.O. (2015). Cavities and Excited States in Proteins. In: Akasaka, K., Matsuki, H. (eds) High Pressure Bioscience. Subcellular Biochemistry, vol 72. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9918-8_12
Download citation
DOI: https://doi.org/10.1007/978-94-017-9918-8_12
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9917-1
Online ISBN: 978-94-017-9918-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)