Abstract
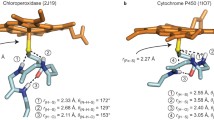
Peroxidases are heme containing enzymes which catalyze the reduction of hydrogen peroxide by different substrates. Peroxidases are characterized by the presence of a polar proximal hydrogen-bond between the Nδ atom of the His fifth ligand and the O atom of a proximal Asp side chain which gives the proximal ligand imidazolate character. To understand the role of the proximal ligand in the catalytic cycle of cytochrome c peroxidase (CCP) different mutants containing Gin, Glu, or Cys in the fifth position has been recently studied and the X-ray structure has been obtained [1].
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Choudhury, K., Sundaramoorthy, M., Hickman, A., Yonetani, T., Woehl, E., Dunn, M. F., & Poulos, T. L. (1994) Crystallographic, kinetic, and spectral studies of cytochrome c peroxidase proximal ligand mutants, J. Biol Chem. 269, 20239–20249.
Hashimoto, S., Teraoka, J., Inubushi, T., Yonetani, T., & Kitagawa, T. (1986) Resonance Raman study on cytochrome c peroxidase and its intermediate, J. Biol. Chem. 261, 11110–11118.
Smulevich, G., Mauro, J. M., Fishel, L. A., English, A. M., Kraut, J., & Spiro, T. G. (1988) Heme pocket interactions in cytochrome c peroxidase studied by site-directed mutagenesis and resonance Raman spectroscopy, Biochemistry 27, 5477–5485.
Hildebrandt, P., English, A. M, & Smulevich, G. (1992) Cytochrome c and cytochrome c peroxidase complex as studied by resonance Raman spectroscopy, Biochemistry 31, 2384–2392.
Smulevich, G., Mantini, A. R., English, A. M., & Mauro, J. M. (1989) Effects of temperature and glycerol on the resonance Raman spectra of cytochrome c peroxidase and selected mutants, Biochemistry 28, 5058–5064.
Finzel, B. C., Poulos, T. L., & Kraut, J. (1984) Crystal structure of yeast cytochrome c peroxidase refined at 1.7 Å resolution, J. Biol. Chem. 259, 13027–13036.
Feis, A., Paoli, M., Marzocchi, M. P., & Smulevich, G. (1994) Spin state and axial ligand bonding in the hydroxide complexes of metmyoglobin, methemoglobin, and horseradish peroxidase at room and low temperatures, Biochemistry 33, 4577–4583.
Takano, T. (1977) Structure of myoglobin refined at 2.0 Å resolution. I. Crystallographic refinement of metmyoglobin from sperm whale, J. Mol. Biol. 110, 537–568.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1995 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Smulevich, G., Neri, F., Willemsen, O., Marzocchi, M.P., Choudhury, K., Poulos, T.L. (1995). Spin and Coordination States of His175Glu Mutant of Cytochrome C Peroxidase at Room and Low Temperatures. In: Merlin, J.C., Turrell, S., Huvenne, J.P. (eds) Spectroscopy of Biological Molecules. Springer, Dordrecht. https://doi.org/10.1007/978-94-011-0371-8_57
Download citation
DOI: https://doi.org/10.1007/978-94-011-0371-8_57
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-010-4166-9
Online ISBN: 978-94-011-0371-8
eBook Packages: Springer Book Archive