Abstract
Plants are astonishingly diverse in how they reproduce sexually, and the study of plant mating systems provides some of the most compelling cases of parallel and independent evolutionary transitions. In this chapter, we review how the massive amount of genomic data being produced is allowing long-standing predictions from ecological and evolutionary theory to be put to test. After a review of theoretical predictions about the importance of considering the genomic architecture of the mating system, we focus on a set of recent discoveries on how the mating system is controlled in a variety of model and non-model species. In parallel, genomic approaches have revealed the complex interaction between the evolution of genes controlling mating systems and genome evolution, both genome-wide and in the mating system control region. In several cases, major transitions in the mating system can be clearly associated with important ecological changes, hence illuminating an important interplay between ecological and genomic approaches. We also list a number of major unsolved questions that remain for the field, and highlight foreseeable conceptual developments that are likely to play a major role in our understanding of how plant mating systems evolve in Nature.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
References
Acosta IF, Laparra H, Romero SP, Schmelz E, Hamberg M, Mottinger JP, Moreno MA, Dellaporta SL (2009) Tasselseed1 is a lipoxygenase affecting jasmonic acid signaling in sex determination of maize. Science 323:262–265
Al-Shehbaz IA, O’Kane SLJ (2002) Taxonomy and phylogeny of Arabidopsis (Brassicaceae). In: Somerville CR, Meyerowitz EM (eds) The Arabidopsis book. American Society of Plant Biologists, Rockville
Ashman TL (2003) Constraints on the evolution of males and sexual dimorphism: filed estimates of genetic architecture of reproductive traits in three populations of gynodioecious Fragaria virginiana. Evolution 57:2012–2025
Bachtrog D (2008) The temporal dynamics of processes underlying Y chromosome degeneration. Genetics 179:1513–1525
Baker HG (1955) Self-compatibility and establishment after “long-distance” dispersal. Evolution 9:347–348
Banks JA (2008) MicroRNA, sex determination and floral meristem determinacy in maize. Genome Biol 9:204
Barrett SCH, Shore JS (2008) New insights on heterostyly: comparative biology, ecology and genetics. In: Franklin-Tong V (ed) Self-incompatibility in flowering plants: evolution, diversity and mechanisms. Springer-Verlag, Berlin, pp 3–32
Bechsgaard JS, Castric V, Charlesworth D et al (2006) The transition to self-compatibility in Arabidopsis thaliana and evolution within S-haplotypes over 10 Myr. Mol Biol Evol 23:1741–1750
Beilstein MA, Nagalingum NS, Clements MD et al (2010) Dated molecular phylogenies indicate a miocene origin for Arabidopsis thaliana. Proc Natl Acad Sci 107(43):18724–18728
Bergero R, Forrest A, Kamau E, Charlesworth D (2007) Evolutionary strata on the X chromosomes of the dioecious plant Silene latifolia: evidence from new sex-linked genes. Genetics 175:1945–1954
Bergero R, Forrest A, Charlesworth D (2008) Active miniature transposons from a plant genome and its nonrecombining Y chromosome. Genetics 178:1085–1092
Billiard S, López-Villavicencio M et al (2011) Having sex, yes, but with whom? Inferences from fungi on the evolution of anisogamy and mating types. Biol Rev 86:421–442
Boggs NA, Nasrallah JB, Nasrallah ME (2009) Independent S-locus mutations caused self-fertility in Arabidopsis thaliana. PLoS Genet 5:e1000426
Boualem A, Fergany M, Fernandez R et al (2008) A conserved mutation in an ethylene biosynthesis enzyme leads to andromonoecy in melons. Science 321(5890):836–838
Busch JW, Schoen DJ (2008) The evolution of self-incompatibility when mates are limiting. Trends Plant Sci 13:128–136
Busch JW, Joly S, Schoen DJ (2011) Demographic signatures accompanying the evolution of selfing in Leavenworthia alabamica. Mol Biol Evol 28:1717–1729
Cao J, Schneeberger K, Ossowski S et al (2011) Whole-genome sequencing of multiple Arabidopsis thaliana populations. Nat Genet 43:956–965
Case AL, Ashman TL (2009) Resources and pollinators contribute to population sex ratio bias and pollen limitation in gynodioecious wild strawberry (Fragaria virginiana). Oikos 118:1250–1260
Castric V, Bechsgaard J, Grenier S et al (2010) Molecular evolution within and between self-incompatibility specificities. Mol Biol Evol 27:11–20
Cegan R, Vyskot B, Kejnovsky E et al (2012) Genomic diversity in two related plant species with and without sex chromosomes – Silene latifolia and S. vulgaris. PLoS ONE 7:e31898
Chantha SC, Herman AC, Platts A, Vekemans X, Schoen DJ (2013) Secondary evolution of self-incompatibility in the mustard genus Leavenworthia. PLoS Biol 11(5):e1001560
Charlesworth D (2002a) Plant sex determination and sex chromosomes. Heredity 88:94–101
Charlesworth D (2002b) What maintains male-sterility factors in plant populations. Heredity 89:408–409
Charlesworth D (2003) Effects of inbreeding on the genetic diversity of populations. Phil Trans R Soc Lond B 358:1051–1070
Charlesworth D (2006) Balancing selection and its effects on sequences in nearby genome regions. PLoS Genet 2:e64
Charlesworth D (2010) Don’t forget the ancestral polymorphisms. Heredity 105:509–510
Charlesworth B, Charlesworth D (1978) A model for the evolution of dioecy and gynodioecy. Am Nat 112:975–997
Charlesworth D, Charlesworth B (1979) Evolution and breakdown of S-allele systems. Heredity 43:41–55
Charlesworth D, Charlesworth B (1987) Inbreeding depression and its evolutionary consequences. Annu Rev Ecol Syst 18:237–268
Charlesworth D, Charlesworth B (1990) Inbreeding depression with heterozygote advantage and its effect on selection for modifiers changing the outcrossing rate. Evolution 44:870–888
Charlesworth B, Charlesworth D (2000) The degeneration of Y chromosomes. Phil Trans R Soc Lond B 355:1563–1572
Charlesworth B, Charlesworth D (2010a) Elements of evolutionary genetics. Roberts and Company Publishers, Greenwood Village
Charlesworth D, Charlesworth B (2010b) Evolutionary biology: the origins of two sexes. Current Biol 20:R520
Charlesworth D, Willis JH (2009) The genetics of inbreeding depression. Nat Rev Genet 10:783–796
Charlesworth D, Wright SI (2001) Breeding systems and genome evolution. Curr Opin Genet Dev 11:685–690
Charlesworth D, Morgan MT, Charlesworth B (1993) Mutation accumulation in finite outbreeding and inbreeding populations. Genet Res 61:39–56
Charlesworth D, Charlesworth B, Morgan MT (1995) The pattern of neutral molecular variation under the background selection model. Genetics 141:1619–1632
Charlesworth D, Vekemans X, Castric V, Glémin S (2005) Plant self-incompatibility systems: a molecular evolutionary perspective. New Phytol 168:61–69
Charnov EL (1982) The theory of sex allocation. Princeton University Press, Princeton
Chookajorn T, Kachroo A, Ripoll DR, Clark AG, Nasrallah JB (2003) Specificity determinants and diversification of the Brassica self-incompatibility pollen ligand. Proc Natl Acad Sci USA 101:911–917
Darracq A, Varré JS, Maréchal-Drouard L et al (2011) Structural and content diversity of mitochondrial genome in beet: a comparative genomic analysis. Genome Biol Evol 3:723–736
Darwin C (1876) The effects of cross and self fertilisation in the vegetable kingdom. John Murray, London
Darwin C (1877) The different forms of flowers on plants of the same species. John Murray, London
de Graaf BH, Vatovec S, Juárez-Díaz JA, Chai L, Kooblall K, Wilkins KA, Zou H, Forbes T, Franklin FC, Franklin-Tong VE (2012) The Papaver self-incompatibility pollen S-determinant, PrpS, functions in Arabidopsis thaliana. Curr Biol 22(2):154–159
de la Chaux N, Tsuchimatsu T, Shimizu KK, Wagner A (2012) The predominantly selfing plant Arabidopsis thaliana experienced a recent reduction in transposable element abundance compared to its outcrossing relative Arabidopsis lyrata. Mob DNA 3:2
Diggle PK, Di Stilio VS, Gschwend AR, Golenberg EM, Moore RC, Russell JRW, Sinclair JP (2011) Multiple developmental processes underlie sex differentiation in angiosperms. Trends Genet 27(9):368–376
Dufaÿ M, Billard E (2012) How much better are females? The occurrence of female advantage, its proximal causes and its variation within and among gynodioecious species. Ann Bot 109:505–519
Dufay M, Cuguen J, Arnaud JF, Touzet P (2009) Sex ratio variation among gynodioecious populations of sea beet: can it be explained by frequency-dependent selection? Evolution 63:1483–1497
Eckert CG, Ozimec B, Herlihy CR, Griffin CA, Routley MB (2009) Floral morphology mediates temporal variation in the mating system of a self-compatible plant. Ecology 90(6):1540–1548
Ehlers BK, Schierup MH (2008) When gametophytic self-incompatibility meets gynodioecy. Genet Res Camb 90:27–35
Entani T, Iwano M, Shiba H et al (2003) Comparative analysis of the self-incompatibility (S-) locus region of Prunus mume: identification of a pollen-expressed F-box gene with allelic diversity. Genes Cells 8:203–213
Escobar JS, Cenci A, Bolognini J et al (2010) An integrative test of the dead-end hypothesis of selfing evolution in Triticeae (Poaceae). Evolution 64:2855–2872
Ferrer MM, Good SV (2012) Self-sterility in flowering plants: preventing self-fertilization increases family diversification rates. Ann Bot 110(3):535–553
Ferris P, Olson B, Hoff PD et al (2010) Evolution of an expanded sex-determining locus in Volvox. Science 328:351–354
Fisher RA (1941) Average excess and average effect of a gene substitution. Ann Eugen 11:53–63
Fishman L, Kelly AJ, Willis JH (2002) Minor quantitative trait loci underlie floral traits associated with mating system divergence in Mimulus. Evolution 56(11):2138–2155
FitzJohn RG (2010) Quantitative traits and diversification. Syst Biol 59:619–633
Foxe JP, Slotte T, Stahl EA, Neuffer B, Hurkad H, Wright SI (2009) Recent speciation associated with the evolution of selfing in Capsella. Proc Natl Acad Sci USA 106(13):5241–5245
Frank SA (1989) The evolutionary dynamics of cytoplasmic male-sterility. Am Nat 133:345–376
Fujimoto R, Okazaki K, Fukai E, Kusaba M, Nishio T (2006) Comparison of the genome structure of the self-incompatibility (S) locus in inter-specific pairs of S haplotypes. Genetics 173:1157–1167
Fukai E, Fujimoto R, Nishio T (2003) Genomic organization of the S core region and the S flanking regions of a class-II S haplotype in Brassica rapa. Mol Gen Genomics 269:361–369
Gervais CE, Castric V, Ressayre A, Billiard S (2011) Origin and diversification dynamics of self-incompatibility haplotypes. Genetics 188:625–636
Glémin S (2007) Mating systems and the efficacy of selection at the molecular level. Genetics 177:905–916
Glémin S, Galtier N (2012) Genome evolution in outcrossing versus selfing versus asexual species. Methods Mol Biol 855:311–335
Glémin S, Ronfort J (2013) Adaptation and maladaptation in selfing and outcrossing species: new mutations versus standing variation. Evolution 67:224–240
Glémin S, Bazin E, Charlesworth D (2006) Impact of mating systems on patterns of sequence polymorphism in flowering plants. Proc R Soc Land B 273:3011–3019
Goldberg EE, Igic B (2012) Tempo and mode in plant breeding system evolution. Evolution 66:3701–3709
Goldberg EE, Kohn JR, Lande R et al (2010) Species selection maintains self-incompatibility. Science 330:493–495
Goubet PM, Bergès H, Bellec A et al (2012) Contrasted patterns of molecular evolution in dominant and recessive self-incompatibility haplotypes in Arabidopsis. PLoS Genet 8:e1002495
Gouyon PH, Vichot F, Van Damme JMM (1991) Nuclear-cytoplasmic male sterility: single point equilibria versus limit cycles. Am Nat 137:498–514
Graham SW, Barrett SCH (2004) Phylogenetic reconstruction of the evolution of stylar polymorphisms in Narcissus (Amaryllidaceae). Am J Bot 91:1007–1021
Guo YL, Bechsgaard JS, Slotte T, Neuffer B, Lascoux M, Weigel D, Schierup MH (2009) Recent speciation of Capsella rubella from Capsella grandiflora, associated with loss of self-incompatibility and an extreme bottleneck. Proc Natl Acad Sci USA 106(13):5246–5251
Guo Y, Zhao X, Lanz C, Weigel D (2011) Evolution of the S-locus region in Arabidopsis thaliana relatives. Plant Physiol 157:937–946
Haudry A, Cenci A, Ravel C, Bataillon T, Brunel D, Poncet C, Hochu I, Poirier S, Santoni S, Glémin S, David J (2007) Grinding up wheat: a massive loss of nucleotide diversity since domestication. Mol Biol Evol 24:1506–1517
Haudry A, Cenci A, Guilhaumon C et al (2008) Mating system and recombination affect molecular evolution in four Triticeae species. Genet Res 90:97–109
Heilbuth JC, Ilves KL, Otto SP (2001) The consequences of dioecy for seed dispersal: modeling the seed-shadow handicap. Evolution 55:880–888
Husse L, Billiard S, Lepart J, Vernet P, Saumitou-Laprade P (2013) A one-locus model of androdioecy in a context with two homomorphic self-incompatibility groups: expected vs. observed male frequencies. J Evol Biol 26(6):1269–1280
Igic B, Lande R, Kohn JR (2008) Loss of self-incompatiblity and its evolutionary consequences. Int J Plant Sci 169:93–104
Indriolo E, Tharmapalan P, Wright SI, Goring DR (2012) The ARC1 E3 Ligase gene is frequently deleted in self-compatible Brassicaceae species and has a conserved role in Arabidopsis lyrata self-pollen rejection. Plant Cell 24:4607–4620
Ingvarsson PK (2002) A metapopulation perspective on genetic diversity and differentiation in partially self-fertilizing plants. Evolution 56:2368–2373
Ingvarsson PK, Taylor DR (2002) Genealogical evidence for epidemics of selfish genes. Proc Natl Acad Sci USA 99:11265–11269
Käfer J, Talianov M, Bigot T et al (2013) Patterns of molecular evolution in dioecious and non-dioecious Silene. J Evol Biol 26:335–346
Kaplan NL, Hudson RR, Langley CH (1989) The hitchhiking effect revisited. Genetics 123:887–899
Kim M, Cui ML, Cubas P, Gillies A, Lee K, Chapman MA, Abbott RJ, Coen E (2008) Regulatory genes control a key morphological and ecological trait transferred between species. Science 322(5904):1116–1119
Kubo K, Entani T, Takara A, Wang N, Fields AM, Hua Z, Toyoda M, Kawashima S, Ando T, Isogai A, Kao TH, Takayama S (2010) Collaborative non-self recognition system in S-RNase-based self-incompatibility. Science 330(6005):796–799
Lahiani E, Dufaÿ M, Castric V, Le Cadre S, Charlesworth D, Van Rossum F, Touzet P (2013) Disentangling the effects of mating systems and mutation rates on cytoplamic diversity in gynodioecious Silene nutans and dioecious Silene otites. Heredity 111:157–164
Lahn BT, Page DC (1999) Four evolutionary strata on the human X chromosome. Science 286:964–967
Liu Z, Moore PH, Ma H et al (2004) A primitive Y chromosome in papaya marks incipient sex chromosome evolution. Nature 427:348–352
Lloyd DG (1975) The maintenance of gynodioecy and androdioecy in angiosperms. Genetica 45:325–339
Lloyd DG (1977) Genetic and phenotypic models of natural selection. J Theor Biol 69:543–560
Lloyd DG (1979) Evolution towards dioecy in heterostylous populations. Plant Syst Evol 131:71–80
Lloyd DG (1980) The distribution of gender in four angiosperm species illustrating two evolutionary pathways to dioecy. Evolution 34:123–134
Lloyd DG, Webb CJ (1992) The evolution of heterostyly. In: Barrett SCH (ed) Evolution and function of heterostyly. Springer, Berlin/Heidelberg/New York, pp 151–178
Lockton S, Gaut BS (2010) The evolution of transposable elements in natural populations of self-fertilizing Arabidopsis thaliana and its outcrossing relative Arabidopsis lyrata. BMC Evol Biol 10:10
Lynch M, Conery J, Burger R (1995a) Mutation accumulation and the extinction of small populations. Am Nat 146:489–518
Lynch M, Conery J, Burger R (1995b) Mutational meltdowns in sexual population. Evolution 49:1067–1080
Maddison WP, Midford PE, Otto SP (2007) Estimating a binary character’s effect on speciation and extinction. Syst Biol 56:701–710
Marais G (2003) Biased gene conversion: implications for genome and sex evolution. Trends Genet 19:330–338
Marais G, Charlesworth B, Wright SI (2004) Recombination and base composition: the case of the highly self-fertilizing plant Arabidopsis thaliana. Genome Biol 5:R45
Martin A, Orgogozo V (2013) The loci of repeated evolution: a catalog of genetic hotspots of phenotypic variation. Evolution 67(5):1235–1250
Martin A, Troadec C, Boualem A, Rajab M, Fernandez R, Morin H, Pitrat M, Dogimont C, Bendahmane A (2009) A transposon-induced epigenetic change leads to sex determination in melon. Nature 461(7267):1135–1138
Mayrose I, Barker MS, Otto SP (2010) Probabilistic models of chromosome number evolution and the inference of polyploidy. Syst Biol 59:132–144
McDaniel SF, Atwood J, Burleigh JG (2013) Recurrent evolution of dioecy in bryophytes. Evolution 67:567–572
Ming R, Bendahmane A, Renner SS (2011) Sex chromosomes in land plants. Annu Rev Plant Biol 62:485–514
Modliszewski JL, Willis JH (2012) Allotetraploid Mimulus sookensis are highly interfertile despite independent origins. Mol Ecol 21:5280–5298
Morgan MT (2001) Transposable element number in mixed mating populations. Genet Res 77:261–275
Muyle A, Zemp N, Deschamps C et al (2012) Rapid de novo evolution of X chromosome dosage compensation in Silene latifolia, a plant with young sex chromosomes. PLoS Biol 10:e1001308
Naithani S, Chookajorn T, Ripoll DR, Nasrallah JB (2007) Structural modules for receptor dimerization in the S-locus receptor kinase extracellular domain. Proc Natl Acad Sci USA 104:12211–12216
Nasrallah ME, Liu P, Nasrallah JB (2002) Generation of self-incompatible Arabidopsis thaliana by transfer of two S locus genes from A. lyrata. Science 297:247–249
Nasrallah M, Liu P, Sherman-Broyles S, Boggs N, Nasrallah J (2004) Natural variation in expression of self-incompatibility in Arabidopsis thaliana: implications for the evolution of selfing. Proc Natl Acad Sci USA 101:16070–16074
Nasrallah JB, Liu P, Sherman-Broyles S, Schmidt R, Nasrallah ME (2007) Epigenetic mechanisms for breakdown of self-incompatibility in interspecific hybrids. Genetics 175:1965–1973
Ness RW, Wright SI, Barrett SCH (2010) Mating-system variation, demographic history and patterns of nucleotide diversity in the tristylous plant Eichhornia paniculata. Genetics 184:381–392
Nicolas M, Marais G, Hykelova V et al (2005) A gradual process of recombination restriction in the evolutionary history of the sex chromosomes in dioecious plants. PLoS Biol 3:e4
Nordborg M (2000) Linkage disequilibrium, gene trees and selfing: an ancestral recombination graph with partial self-fertilization. Genetics 154:923–929
Nordborg M, Hu TT, Ishino Y et al (2005) The pattern of polymorphism in Arabidopsis thaliana. PLoS Biol 3:1289–1299
Ornduff R (1969) Reproductive biology in relation to systematics. Taxon 18:121–133
Pannell JR, Verdu M (2006) The evolution of gender specialization from dimorphic hermaphroditism: paths from heterodichogamy to gynodioecy and androdioecy. Evolution 60:660–673
Pettengill JB, Moeller DA (2011) Tempo and mode of mating system evolution between incipient Clarkia species. Evolution 66:1210–1225
Porcher E, Lande R (2005a) The evolution of self-fertilization and inbreeding depression under pollen discounting and pollen limitation. J Evol Biol 18:497–508
Porcher E, Lande R (2005b) Loss of gametophytic self-incompatibility with evolution of inbreeding depression. Evolution 59:46–60
Qiu S, Zeng K, Slotte T et al (2011) Reduced efficacy of natural selection on codon usage bias in selfing Arabidopsis and Capsella species. Genome Biol Evol 3:868–880
Renner SS, Ricklefs RE (1995) Dioecy and its correlates in the flowering plants. Am J Bot 82:596–606
Roselius K, Stephan W, Stadler T (2005) The relationship of nucleotide polymorphism, recombination rate and selection in wild tomato species. Genetics 171:753–763
Ross MD (1982) Five evolutionary pathways to subdioecy. Am Nat 119:297–318
Ross-Ibarra J, Wright SI, Foxe JP et al (2008) Patterns of polymorphism and demographic history in natural populations of Arabidopsis lyrata. PLoS ONE 3:e2411
Roze D, Rousset F (2004) Joint effects of self-fertilization and population structure on the mutation load, inbreeding depression and heterosis. Genetics 167:1001–1015
Saumitou-Laprade P, Vernet P, Vassiliadis C, Hoareau Y, de Magny G, Dommée B, Lepart J (2010) A self-incompatibility system explains high male frequencies in an androdioecious plant. Science 327(5973):1648–1650
Schierup MH, Vekemans X, Christiansen FB (1997) Evolutionary dynamics of sporophytic self-incompatibility alleles in plants. Genetics 147:835–846
Schoen DJ, Brown AHD (1991) Intraspecific variation in population gene diversity and effective population size correlates with the mating system in plants. Proc Natl Acad Sci USA 88:4494–4497
Segawa M, Kishi S, Tatuno S (1971) Sex chromosomes of Cycas revoluta. Jpn J Genet 46:33–39
Shimizu KK, Cork JM, Caicedo AL et al (2004) Darwinian selection on a selfing locus. Science 306:2081–2084
Shimizu KK, Shimizu-Inatsugi R, Tsuchimatsu T, Purugganan MD (2008) Independent origins of self-compatibility in Arabidopsis thaliana. Mol Ecol 17:704–714
Sicard A, Stacey N, Hermann K, Dessoly J, Neuffer B, Bäurle I, Lenhard M (2011) Genetics, evolution, and adaptive significance of the selfing syndrome in the genus Capsella. Plant Cell 23(9):3156–3171
Slotte T, Foxe JP, Hazzouri KM, Wright SI (2010) Genome-wide evidence for efficient positive and purifying selection in Capsella grandiflora, a plant species with a large effective population size. Mol Biol Evol 27:1813–1821
Slotte T, Hazzouri KM, Stern D, Andolfatto P, Wright SI (2012) Genetic architecture and adaptive significance of the selfing syndrome in Capsella. Evolution 66(5):1360–1374
Slotte T, Hazzouri KM, Ågren JA, Koenig D, Maumus F, Guo Y, Steige K, Platts AE, Escobar JS, Newman LK, Wang W, Mandakova T, Vello E, Smith SM, Steffen J, Takuno S, Brandvain Y, Coop G, Andolfatto P, Hu TT, Blanchette M, Clark RM, Queseville H, Nordborg M, Gaut BS, Lysak MA, Jenkins J, Grimwood J, Prochnick S, Shu S, Rokhsar D, Schmutz J, Weigel D, Wright SI (2013) The Capsella rubella genome and the genomic consequences of rapid mating system evolution. Nat Genet 45:831–835
Spigler RB, Lewers KS, Main DS, Ashman TL (2008) Genetic mapping of sex determination in a wild strawberry, Fragaria virginiana, reveals earliest form of sex chromosome. Heredity 101:507–517
St Onge KR, Kallman T, Slotte T et al (2011) Contrasting demographic history and population structure in Capsella rubella and Capsella grandiflora, two closely related species with different mating systems. Mol Ecol 20:3306–3320
Stadler T (2011) Inferring speciation and extinction processes from extant species data. Proc Natl Acad Sci USA 108:16145–16146
Stadler T, Delph LF (2002) Ancient mitochondrial haplotypes and evidence for intragenic recombination in a gynodioecious plant. Proc Natl Acad Sci USA 99:11730–11735
Stebbins GL (1957) Self fertilization and population variability in higher plants. Am Nat 91:337–354
Stebbins GL (1974) Flowering plants: evolution above the species level. Harvard University Press, Cambridge
Takayama S, Isogai A (2005) Self-incompatibility in plants. Annu Rev Plant Biol 56:467–489
Takebayashi N, Morrell PL (2001) Is self-fertilization an evolutionary deed end? Revisiting an old hypothesis with genetic theories and a macroevolutionary approach. Am J Bot 88:1143–1150
Tam SM, Causse M, Garchery C et al (2007) The distribution of copia-type retrotransposons and the evolutionary history of tomato and related wild species. J Evol Biol 20:1056–1072
Tang C, Toomajian C, Sherman-Broyles S, Plagnol V, Guo YL et al (2007) The evolution of selfing in Arabidopsis thaliana. Science 317:1070–1072
Tantikanjana T, Rizvi N, Nasrallah ME, Nasrallah JB (2009) A dual role for the S-locus receptor kinase in self-incompatibility and pistil development revealed by an Arabidopsis rdr6 mutation. Plant Cell 21(9):2642–2654
Torices R, Méndez M, Gomez MJ (2011) Where do monomorphic sexual systems fit in the evolution of dioecy? Insights from the largest family of angiosperms. New Phytol 190:234–248
Touzet P (2012) Mitochondrial genome evolution and gynodioecy. In: Marechal-Drouard L (ed) Advances in botanical research, vol 63. Academic Press/Elsevier Science, London, pp 71–98
Touzet P, Budar F (2004) Unveiling the molecular arms race between two conflicting genomes in cytoplasmic male sterility? Trends Plant Sci 9:568–570
Touzet P, Delph LF (2009) The effect of breeding system on polymorphism in mitochondrial genes of Silene. Genetics 181:631–644
Tsuchimatsu T, Suwabe K, Shimizu-Inatsugi R et al (2010) Evolution of self-compatibility in Arabidopsis by a mutation in the male specificity gene. Nature 464:1342–1346
Tsuchimatsu T, Kaiser P, Yew C-L, Bachelier JB, Shimizu KK (2012) Recent loss of self-incompatibility by degradation of the male component in allotetraploid Arabidopsis kamchatica. PLoS Genet 8(7):e1002838
Uyenoyama MK (2003) Genealogy-dependent variation in viability among self-incompatibility genotypes. Theor Popul Biol 63:281–293
Uyenoyama M, Zhang Y, Newbigin E (2001) On the origin of self-incompatibility haplotypes: transition through self-compatible intermediates. Genetics 157:1805–1817
Vamosi JC, Otto SP (2002) When looks can kill: the evolution of sexually dimorphic floral display and the extinction of dioecious plants. Proc R Soc Lond B 269:1187–1194
van Doorn GS, Kirkpatrick M (2007) Turnover of sex chromosomes induced by sexual conflict. Nature 449:909–912
Vekemans X, Slatkin M (1994) Gene and allelic genealogies at a gametophytic self-incompatibility locus. Genetics 137:1157–1165
Wang J, Naa JK, Yub Q et al (2012) Sequencing papaya X and Y chromosomes reveals molecular basis of incipient sex chromosome evolution. Proc Natl Acad Sci USA 109:13710–13715
Wright SI, Schoen DJ (1999) Transposon dynamics and the breeding system. Genetica 107:139–148
Wright SI, Le QH, Schoen DJ, Bureau TE (2001) Population dynamics of an Ac-like transposable element in self- and crosspollinating Arabidopsis. Genetics 158:1279–1288
Wright SI, Lauga B, Charlesworth D (2002) Rates and patterns of molecular evolution in inbred and outbred Arabidopsis. Mol Biol Evol 19:1407–1420
Wright S, Ness RW, Foxe JP, Barrett SC (2008) Genomic consequences of outcrossing and selfing in plants. Int J Plant Sci 169:105–118
Acknowledgements
We thank two anonymous reviewers for helpful comments. Our work on plant mating systems is supported by the French Agence Nationale de la Recherche (ANR-11-BSV7- 013-03 and ANR 11 JSV7 008 01) and by the Région Nord Pas de Calais.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Glossary
- Hermaphroditism
-
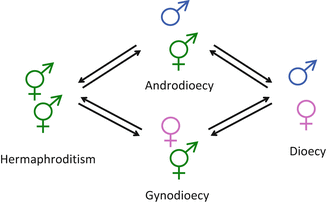
Hermaphroditism is the simultaneous coexistence of male and female reproductive organs on the same (co-sexual) individual (Fig. 2.1). Hermaphroditism is believed to be the ancestral state in Angiosperms, and the defining organ of Angiosperms (the flower) is itself a hermaphroditic organ, producing both pollen and ovules.
- Selfing versus outcrossing
-
A major consequence of hermaphroditism is the potential for self-fertilization, the most extreme form of inbreeding.
- Self-incompatibility (SI)
-
A genetic system promoting allogamy in many plants. There are two different types of SI, homomorphic and heteromorphic. In homomorphic SI, the different groups of mating partners differ by the type of recognition proteins they produce, but remain morphologically undistinguishable. In heteromorphic SI (e.g. heterostyly), the two (distyly) or three (tristyly) self- and within-morph incompatible mating groups typically differ by style length, anther height and pollen size.
- Inbreeding depression
-
Inbreeding depression is the decrease of fitness of offspring produced by inbred parents relatively to those produced by unrelated parents.
- Pollen limitation
-
A plant is pollen-limited if it does not receive enough pollen to fertilize all its ovules. Pollen limitation thus leads to a reduction in reproductive output through the female function.
- Pollen discounting
-
The loss of male reproduction in cross-fertilization due to the decrease of exported pollen, which occurs especially in autogamous species.
- Gynodioecy (resp. androdioecy)
-
Gynodioecy (resp. androdioecy) is a mating system whereby hermaphrodite individuals coexist with female (resp. male) individuals (Fig. 2.1).
- Dioecy
-
Dioecy is the separation of sexual functions in specialized (male and female) individuals (Fig. 2.1). Species in which some individuals with incomplete sexual specialization occur along with strictly unisexual individuals are termed subdioecious.
- Monoecious
-
Monoecious species are composed of hermaphrodites only, but in which male and female flowers are separated on each individual.
In gynomonoecious species, some individuals produce female-only flowers in variable proportion along with hermaphroditic flowers, while in andromonoecious species, some individuals produce male-only flowers along with hermaphroditic flowers.
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Castric, V., Billiard, S., Vekemans, X. (2014). Trait Transitions in Explicit Ecological and Genomic Contexts: Plant Mating Systems as Case Studies. In: Landry, C., Aubin-Horth, N. (eds) Ecological Genomics. Advances in Experimental Medicine and Biology, vol 781. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7347-9_2
Download citation
DOI: https://doi.org/10.1007/978-94-007-7347-9_2
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7346-2
Online ISBN: 978-94-007-7347-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)


